Maintenance of integrity of the nervous system with gangliosides | ||||||||||||||||
 |
While acidic glycosphingolipids, gangliosides, are abundantly expressed in nervous tissues, their functions have long been unclear. However, studies using various knock-out mice of ganglioside synthase genes have revealed their involvement in maintaining the healthy condition of the nervous systems. Gangliosides, particularly those with elongated sugar chains (GM1, GD1a, GD1b, GT1b, etc.), are thought to be essential for neural development and functions due to their unique expression in the nervous system. In 1996, Takamiya et al. generated GM2/GD2 synthase gene knockout (KO) mice lacking complex gangliosides highly expressed in neural tissues. Although these KO mice were expected to have significant defects in their nervous systems, they developed normally and had normal neural tissue development. However, with aging, neurodegeneration was observed in areas like the sciatic nerve and spinal cord posterior horn, and proliferation of glial cells was also observed in these KO mice (1). The absence of complex gangliosides in these KO mice might alter the expression of alternative gangliosides such as GD3 and be compensating for the functions of gangliosides in the nervous system. In 2002, Okada et al. reported the generation of GD3 synthase gene KO mice, lacking b-series gangliosides beyond GD3. Although these mice showed normal development including neural tissues, their capability for nerve regeneration following hypoglossal nerve transection was reduced (2). Since the levels of neurodegeneration in these ganglioside synthase gene KO mice were milder than expected, double KO (DKO) mice were generated by crossing GM2/GD2 synthase gene KO mice with GD3 synthase gene KO mice. All gangliosides except GM3 have been lost in these DKO mice (3). These mice showed sudden death after 12 weeks of birth and significant neural degeneration including movement abnormalities and loss of Purkinje cells from an early age. Furthermore, inflammatory reaction such as complement activation, increased expression levels of inflammatory cytokines, and proliferation of astrocytes and microglia were induced in the central nervous tissues of DKO mice. It has been found that this inflammatory response accompanied with complement activation is caused by dysregulation of complement regulatory factors (DAF and CD59) (4).
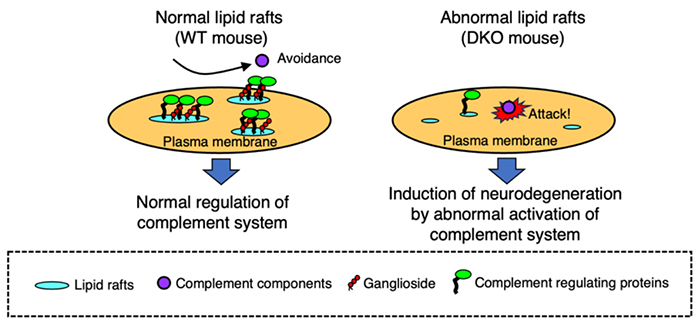
Complement regulatory factors are GPI-anchored proteins that are localized in lipid rafts. In the central nervous systems of DKO mice, abnormalities in lipid raft construction are induced due to gangliosides deficiency, leading to abnormal localization of DAF and CD59, and uncontrolled complement activation. Therefore, it was suggested that neurodegeneration associated with aging in the DKO mice gradually induced by abnormal activation of the complement systems (Fig 1). This was supported by creating triple KO (TKO) mice, lacking gangliosides and complement C3, where neurodegeneration caused by ganglioside deficiency was alleviated by complement deficiency (4). However, alleviation of the neurodegeneration in TKO mice did not result in a complete reversal, suggesting the existence of complement-independent neurodegeneration mechanisms. Additionally, our experiments using wild-type mice, GM2/GD2 synthetase gene KO mice, GD3 synthase gene KO mice, and DKO mice revealed that the degree of abnormality in lipid raft architecture was significantly affected depending on the level of ganglioside deficiency (5). Thus, it has been found that ganglioside expression in the normal nervous system maintains the integrity of the nervous systems by stabilizing the construction of lipid rafts and controlling the behavior of membrane proteins. However, results from TKO mice suggest that there are other mechanisms for maintenance of the healthy nervous systems mediated by gangliosides. Yao et al. investigated which type of cells are responsible for inducing neurodegeneration caused by ganglioside deficiency using cell type-specific rescue of GM2/GD2 synthase gene in the GM2/GD2 synthase gene KO mice (GalNAcT-/--Tg mice). In these mice, GM2/GD2 synthase gene was specifically rescued in either neurons or glial cells (oligodendrocytes) in the GM2/GD2 synthase gene KO background. As a result, GalNAcT-/--Tg (neuronal) mice maintained neural function similar to wild-type mice, whereas GalNAcT-/--Tg (glial) mice exhibited neurodegeneration similar to GM2/GD2 synthase gene KO mice (6). This result indicated that gangliosides, which are mainly expressed in nerve cells, are important for the maintenance of the nervous systems. However, since the glial cell rescue in this study was specific to oligodendrocytes, the function of gangliosides in astrocytes is still unclear. In summary, the role of gangliosides in the nervous system has gradually become clear using various ganglioside synthase gene knockout mice. but there are still many unknown functions, and further research is anticipated to clarify those functions. Yuhsuke Ohmi
| |||||||||||||||
| References | |
|---|---|
| (1) | Takamiya K, Yamamoto A, Furukawa K, Yamashiro S, Shin M, Okada M, Fukumoto S, Haraguchi M, Takeda N, Fujimura K, Sakae M, Kishikawa M, Shiku H, Furukawa K, Aizawa S: Mice with disrupted GM2/GD2 synthase gene lack complex gangliosides but exhibit only subtle defects in their nervous system. Proc. Natl. Acad. Sci. U S A 93, 10662-10667, 1996 |
| (2) | Okada M, Itoh Mi M, Haraguchi M, Okajima T, Inoue M, Oishi H, Matsuda Y, Iwamoto T, Kawano T, Fukumoto S, Miyazaki H, Furukawa K, Aizawa S, Furukawa K: b-series ganglioside deficiency exhibits no definite changes in the neurogenesis and the sensitivity to Fas-mediated apoptosis but impairs regeneration of the lesioned hypoglossal nerve. J. Biol. Chem. 277, 1633-1636, 2002 |
| (3) | Tajima O, Egashira N, Ohmi Y, Fukue Y, Mishima K, Iwasaki K, Fujiwara M, Inokuchi J, Sugiura Y, Furukawa K, Furukawa K: Reduced motor and sensory functions and emotional response in GM3-only mice: emergence from early stage of life and exacerbation with aging. Behav. Brain Res. 198, 74-82, 2009 |
| (4) | Ohmi Y, Tajima O, Ohkawa Y, Mori A, Sugiura Y, Furukawa K, Furukawa K: Gangliosides play pivotal roles in the regulation of complement systems and in the maintenance of integrity in nerve tissues. Proc. Natl. Acad. Sci. U S A 106, 22405-22410, 2009 |
| (5) | Ohmi Y, Tajima O, Ohkawa Y, Yamauchi Y, Sugiura Y, Furukawa K, Furukawa K: Gangliosides are essential in the protection of inflammation and neurodegeneration via maintenance of lipid rafts: elucidation by a series of ganglioside-deficient mutant mice. J. Neurochem. 116, 926-35, 2011 |
| (6) | Yao D, McGonigal R, Barrie JA, Cappell J, Cunningham ME, Meehan GR, Fewou SN, Edgar JM, Rowan E, Ohmi Y, Furukawa K, Furukawa K, Brophy PJ, Willison HJ: Neuronal expression of GalNAc transferase is sufficient to prevent the age-related neurodegenerative phenotype of complex ganglioside-deficient mice. J. Neurosci. 34, 880-891, 2014 |
Mar. 15, 2024