 |
Release of cancer cells from the original portion and decreases in cell-cell adhesion are thought to be the first step in tumor metastasis. E-cadherin is involved in a homophilic type of cell adhesion molecule which acts in Ca-dependent manner. Demethylation of promoter lesion in E-cadherin suppresses expression of E-cadherin mRNA, leading to tumor metastasis in certain cancers. E-cadherin has four potential sites for N-linked oligosaccharides and it has been reported that the oligosaccharides regulate E-cadherin functions. The enzyme GnT-III (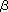 1,4N-acetylglucosaminyltransferase III) catalyzes the addition of a bisecting N-acetylglucosamine (GlcNAc) residue on glycoproteins and serves to suppress the elongation of oligosaccharides. When GnT-III was over-expressed in mouse melanoma cells, experimental metastasis to lung was dramatically suppressed (1). The mechanisms underlying the suppression of metastasis are due to prolongation of E-cadherin turn-over on the cell surface, resulting in increase of its expression (2). However, this mechanism was not observed in human cancers. In the first step of cancer metastasis, cell-cell contacts and aggregation become weak. Another mechanism exists in the alteration of E-cadherin function. E-cadherin is complexed via a special cytoplasmic binding domain with three cytosolic proteins, termed 1,4N-acetylglucosaminyltransferase III) catalyzes the addition of a bisecting N-acetylglucosamine (GlcNAc) residue on glycoproteins and serves to suppress the elongation of oligosaccharides. When GnT-III was over-expressed in mouse melanoma cells, experimental metastasis to lung was dramatically suppressed (1). The mechanisms underlying the suppression of metastasis are due to prolongation of E-cadherin turn-over on the cell surface, resulting in increase of its expression (2). However, this mechanism was not observed in human cancers. In the first step of cancer metastasis, cell-cell contacts and aggregation become weak. Another mechanism exists in the alteration of E-cadherin function. E-cadherin is complexed via a special cytoplasmic binding domain with three cytosolic proteins, termed  -, -, 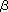 -, -,  -catenins. The elimination of E-cadherin-mediated cell-cell adhesion leads to the decreased expression, deletion or mutational inactivation of either E-cadherin or -catenins. The elimination of E-cadherin-mediated cell-cell adhesion leads to the decreased expression, deletion or mutational inactivation of either E-cadherin or 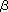 -catenin. -catenin.
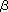 -catenin has been shown to be the common target for signal transduction pathways mediated by the EGF and proto-oncogenes src and Wnt-1. It has recently been proposed that the phosphorylation of a tyrosine residue of -catenin has been shown to be the common target for signal transduction pathways mediated by the EGF and proto-oncogenes src and Wnt-1. It has recently been proposed that the phosphorylation of a tyrosine residue of 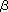 -catenin is associated with cell migration (3). Increases in tyrosine-phosphorylated -catenin is associated with cell migration (3). Increases in tyrosine-phosphorylated 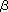 -catenin are thought to contribute to the malignant progression and metastasis of tumor cells. The addition of bisecting GlcNAc residues to E-cadherin suppresses the tyrosine phosphorylation of -catenin are thought to contribute to the malignant progression and metastasis of tumor cells. The addition of bisecting GlcNAc residues to E-cadherin suppresses the tyrosine phosphorylation of 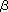 -catenin, leading to maintenance of cell-cell contacts (4). -catenin, leading to maintenance of cell-cell contacts (4).
As a result, a low degree of oligosaccharide branching of E-cadherin may lead to the suppression of tumor progression such as invasion or metastasis. An interesting topic of future research would be to find which oligosaccharide of the four potential sites is important in the above function of E-cadherin and determine how the oligosaccharide regulates the functions of E-cadherin. |
|
|
| References |
(1) |
Yoshimura M, Nishikawa A, Ihara Y, Taniguchi S, Taniguchi N: Suppression lung metastasis of B16 mouse melanoma by N-acetylglucosaminyltransferase III gene transfection. Proc. Natl. Acad. Sci. U. S. A., 92, 8754-8758, 1995 |
|
(2) |
Yoshimura M, Ihara Y, Matsuzawa Y, Taniguchi N: Aberrant glycosylation of E-cadherin enhances cell-cell binding to suppress metastasis. J. Biol. Chem., 271, 13811-13815, 1996 |
|
(3) |
Müller T, Choidas A, Reichmann E, Ullrich A: Phosphorylation and free pool of 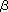 -catenin are regulated by tyrosine kinases and tyrosine phosphatases during epithelial cell migration. J. Biol. Chem., 274, 10173-10183, 1999 -catenin are regulated by tyrosine kinases and tyrosine phosphatases during epithelial cell migration. J. Biol. Chem., 274, 10173-10183, 1999 |
|
|
(4) |
Kitada T, Miyoshi E, Noda K, Higashiyama, S, Ihara H, Matsuura N. Hayashi N, Kawata S, Matsuzawa Y, Taniguchi N: The addition of bisecting N-acetylglucosamine residues to E-cadherin down-regulates the tyrosine phosphorylation of 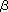 -catenin. J. Biol. Chem., 276, 475-480, 2001 -catenin. J. Biol. Chem., 276, 475-480, 2001 |
|
|
|
|
|



