| Sulfation of Glycolipids | |
|
 |
The sulfate group of sulfated glycolipids (SGLs) is transferred from 3'-phosphoadenosine 5'-phosphosulfate (PAPS) by the catalysis of a sulfotransferase located in the Golgi membranes. Two sulfotransferases acting on different acceptor substrates are involved in the biosynthesis of SGLs. One is cerebroside sulfotransferase (CST: EC 2.8.2.11) that synthesizes sulfatide (galactosylceramide sulfate, SM4s), transferring sulfate onto position 3 of galactose of galactosylceramide (cerebroside). The other is the sulfotransferase that synthesizes HNK-1 epitope (Fig. 1). The former also acts on lactosylceramide and galactosylalkylacylglycerol, synthesizing lactosylceramide sulfate (SM3) and seminolipid (SM4g), respectively. In normal tissues, sulfatide is a major lipid component of brain myelin sheath, and also distributed in renal tubular cells and epithelial cells of the gastrointestinal tract. Seminolipid is abundant in brain during myelination and in spermatogenesis. The turnover of sulfatide in brain and seminolipid in sperm is considerably slower than that of sulfatide in kidney. Glycolipids possessing HNK-1 epitope, SGGL-1 (HSO3-3GlcUb-3Galb-4GlcNAcb-3Galb-4Glcb-1Cer) and SGGL-2 (HSO3-3GlcUb-3Galb-4GlcNAcb-3Galb-4GlcNAcb-3Galb-4Glcb-1Cer) are observed in developing central nerve and cauda equina in peripheral nerve. Since the distribution of SGLs is tissue-specific, their biological role is implicated at the sites where they are expressed. Definitive evidence, however, has not been obtained.
| |
|
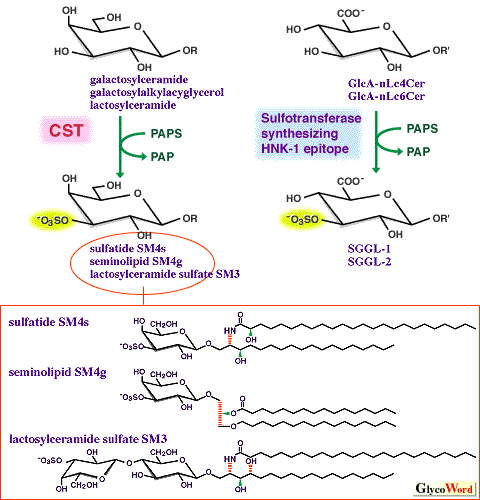
Fig1. The enzyme reaction of two glycolipid sulfotransferases,
and the structures of major sulfated glycolipids.
|
|
|
The biosynthesis of SGLs is active during myelination in brain and spermatogenesis in testis. It changes with the menstrual cycle in uterine endometrium. Vitamin K and butylate activate the biosynthesis. It is also activated when MDCK cells, canine renal tubular cells, are cultured under high osmotic pressure. The detailed mechanisms are unknown.
SGLs accumulate as a consequence of elevated of CST activity in human cancers such as lung cancer, stomach cancer, colon cancer, liver cancer, ovarian cancer, glioma, and kidney cancer. SGLs are expressed in cancer cells themselves. In renal cancer cells, activated protein kinase C and tyrosine kinase stimulate the transcription of CST gene downstream of their intracellular signal transduction system, leading to increase of CST activity. The pathological significance of SGLs expressed in cancer cells remains to be solved.
The CST purified from human renal cancer cells has a molecular weight of 54,000. The primary structure deduced from the nucleotide sequence of human CST cDNA is a type II membrane protein consisting of 423 amino acids. It possesses two N-glycosylation sites. CST shows significant homology to neither the cytosolic sulfotransferases involved in drug metabolism nor the Golgi sulfotransferase functioning in the sulfation of glycosaminoglycans. However, PAPS binding motifs are suggested based on the expected similarity in the three-dimensional structures. Mouse CST also consists of 423 amino acids and its amino acid sequence shows 84% homology compared with that of human CST. On Northern blotting analysis, 1.9 kb and 1.8 kb transcripts are observed in human and mouse, respectively. In mouse organs, CST mRNA is detected in kidney, brain, testis, stomach, small intestine, liver, and lung. The human CST gene is located in chromosome 22.
As regards evolution, SGLs are only distributed in animals of the deutrosome lineage from echinoderms (for instance, sea urchin) to vertebrates. The ancestry of the CST gene and acquired phenotype accompanied by the generation of CST gene are important questions for the future.
| |
|
| Koichi Honke (Osaka Medical Center for Maternal and Child Health, Research Institute) | |
|
|
| References | (1) | Ishizuka, I : Chemistry and functional distribution of sulfoglycolipids. Prog. Lipid Res. 36, 245-319 (1997) (review) |
| (2) | Vos, J P, Lopes-Cardozo, M, Gadella, B M : Metabolic and functional aspects of sulfogalactolipids. Biochim. Biophys. Acta 1211, 125-149 (1994) (review) |
| (3) | Honke, K, Tsuda, M, Hirahara, Y, Ishii, A, Makita, A, Wada, Y : Molecular cloning and expression of cDNA encoding human3'-phosphoadenylysulfate:galactosylceramide 3'-sulfotransferase. J. Biol.Chem. 272, 4864-4868 1997 |
| | |
| |
| Dec.15, 1998 |
|
| |
|
|
|
|



