| Sweet ER Quality Control: The Role of N-glycosylation in Protein Quality Control | |
|
 |
The quality of proteins in cells is monitored by various control mechanisms that are generally connected to degradation pathways. The endoplasmic reticulum (ER) is the port of entry of membrane and secretory proteins into the secretory pathway, and is also equipped with the machinery, called the ER quality control mechanism (1). Recent advances in the area of ER quality control and of lectin-based ER molecular chaperones (calnexin and calreticulin) begin to provide the long-waited answer for a fundamental glycobiology question|why do cells have to transfer rather intricate N-glycans to nascent proteins in the ER?
ER quality control machinery
Synthesis of secretory and membrane proteins is initiated, when ribosomes that are translating messenger RNA dock the ER membrane and insert peptide chains into the ER lumen. Ribosomal docking sites are called translocons--aqueous pores composed of multiple proteins including the Sec61p complex (Fig. A-1). In the ER lumen, nascent peptide chains acquire their mature tertiary structure with the aid of various molecular chaperones and protein-disufide-isomerases (Fig. A-2, 3, 4). Some of the folded proteins further assemble to form multi-subunit complexes. Once the proteins acquire the maturely folded structures that are necessary for exerting their functions, proteins are transported to the Golgi apparatus then delivered to the cell surface, where secretory proteins are secreted and membrane proteins are expressed on the surface of plasma membrane.
If nascent proteins fail to fold or to oligomerize correctly, such misfolded proteins could form aggregation or inhibit cellular mechanisms. ER quality control mechanisms ensure that such misfolded, non-productive proteins are not transported to distal secretory compartments beyond the ER. Recent papers describe the detailed fate of misfolded proteins (1). The misfolded proteins are deported to the cytoplasm (Fig. A-5). This retrograde translocation is achieved by transporting peptides through a pore on the ER membrane that may be composed in part of the Sec61p translocation complex (Fig. A-5). In the cytoplasm, N-linked glycans attached to the glycoproteins are removed by N-glycanases and exiled proteins are degraded into small peptides by cytoplasmic proteasomes that have multiple proteolytic activities (1). It is also indicated that various ER chaperones (calnexin, calreticulin, Bip, etc.) actively participate in the recognition and/or retention of the mis/unfolded proteins (1, 2). As folding of nascent peptide chains is suggested to commence during translation, the progress of folding must be also monitored closely from the birth of peptide chains. In fact, it is suggested that the fate of misfolded nascent peptides could be established by ubiquitination machinery while nascent peptides are still elongating on ribosomes (3).
Involvement of defined oligosaccharide structures on nascent proteins in the ER quality control system
The majority of nascent peptides in the ER are N-glycosylated with high mannose type oligosaccharide chains (Glucose(Glc)3-Mannose(Man)9-N-Acetylglucosamine(GlcNAc)2) (Fig. B-a) on asparagine residues of growing peptides. Immediately thereafter, glucose moieties are trimmed by the sequential actions of glucosidase I and II (Fig. B-1, 2).
There are at least three observations that had not been well understood in the area of N-glycan processing in the ER.
1) Why do cells have to transfer rather intricate glycan structures, Glc3Man9GlcNAc2, to nascent peptide chains even though all glucose moieties on those glycans are removed in the ER?
2) Why is the presence of monoglucosyl oligosaccharides (Glc1Man5-9GlcNAc2) on some proteins important for the efficient secretion or expression on the cell surface?
3) Why does the ER maintain the active re- and de-glucosylation cycle that does not have any obvious effects on the processing of N-glycans?
The phenomena mantioned above can be biologically defined by the recent progress made in the study of a new class of ER molecular chaperones (calnexin and calreticulin) and of the re- and de-glucosylation cycle. It has been demonstrated that the re- and de-glucosylation cycle produces unique monoglucosylated glycans attached to un/misfolded proteins, which are then recognized by calnexin and calreticulin. These interactions with calnexin/calreticulin are suggested to promote correct folding and oligomerization of many newly synthesized glycoproteins.
Lectin-like molecular chaperones and the re- and de-glycosylation cycle
Calnexin is a type I transmembrane protein located in the ER, while its homologue, calreticulin is a soluble protein of the ER lumen. Both have calcium-dependent lectin activity with high affinity towards the monoglucosylated N-linked glycans, Glc1Man5-9GlcNAc2, which arise as transient intermediates during the processing of oligosaccharides in the ER (Fig. B-c,e). Calnexin and calreticulin associate transiently with numerous newly synthesized glycoproteins in ER. Such interactions with calnexin/calreticulin have been indicated to promote productive folding and oligomerization of proteins. Therefore both ER lectins are categorized as molecular chaperones.
Nascent proteins acquire their ternary structure by cycle of association and dissociation with molecular chaperones (on- and off- cycle). Most of molecular chaperones except calnexin and calreticulin directly bind to the unfolded peptide domains of proteins, and ATP hydrolysis is the driving force for the on-and off- cycle. In contrast, it is proposed that the on- and off-cycle with calnexin/calreticulin is mediated with their lectin activities together with the ER re- and deglucosylation cycle (Fig. B, purple square). First, UDP-glucose: glycoprotein glucosyltransferase is suggested to transfer one glucose moiety on N-linked glycans attached to non-native glycoproteins (Fig. B-5). As a result, the proteins acquire the defined glycans, Glc1Man5-9GlcNAc2, which bind to calnexin/calreticulin (Fig. B, pink square). Thereafter, the added glucose moieties are again removed by glucosidase II (Fig. B-4,5), resulting in the release of glycoproteins from calnexin/calreticulin. This model predicts that glycoproteins stay in the on- and off-cycle until they fold correctly and no longer qualify as substrates for reglucosylation by glycoprotein glucosyltransferase. In mammalian cells, it is suggested that the de- and reglucosylation cycle promotes the folding of newly synthesized glycoprotein through the cyclic interaction of calnexin/calreticulin. It is also indicated that retention of mis/unfolded proteins is achieved partly by the association with calnexin that is located on the ER membrane.
Future perspectives
Recent advances in the understanding of the functions of lectin-like molecular chaperones has established that calnexin and calreticulin are a part of the ER quality machinery that monitors the progress of folding of nascent proteins. However, investigation of the detailed functions of calnexin/calreticulin is only beginning. There are many questions to be resolved.
| 1) | It is controversial whether calnexin/calreticulin binds the unfolded peptide domains directly. If the association of glycoproteins and calnexin/calreticulin is only mediated by N-glycans on proteins, how is the molecular chaperone activity exerted by these lectins? Do other molecules associated with calnexin/calreticulin (e.g. Erp57, a protein-disufide-isomerase) chaperone productive folding? |
| |
| 2) | Is deglucosylation by glucosidase II the only way to release proteins from calnexin/calreticulin? Are any other mechanisms, such as ATP hydrolysis or manipulation of calcium concentration in the microenvironment, involved in the dissociation of proteins from calnexin/calreticulin? |
| |
| 3) | A recent report suggests that calnexin is involved in the retrograde transport of misfolded protein to cytoplasm. Does monoglucosylated N-glycan actively participate in this process? |
It is now recognized that ER quality control machinery closely participates in the pathogenesis of disease. One example is cystic fibrosis, a lethal hereditary exocrinopathy affecting one in two thousand children among the Caucasian population. The vast majority of cystic fibrosis patients are linked to a single aminoacid deletion on a chloride channel called CFTR. Although the mutant CFTR has chloride conductance activity, ER quality control machinery retains the mutant CFTR in the ER. This retention results in lack of chloride conductance activity on the cellular surface, inducing various symptoms of cystic fibrosis. When a chemical treatment releases mutant CFTR from the ER,the cell is able to transport mutant CFTR to the cell surface and conduct chloride transport at the cell surface (5). Such treatment has an important clinical implication for cystic fibrosis. Therefore, understanding the functional roles of N-glycans in ER quality control machinery should shed light on remedies of various ER quality control-related diseases.
| |
|
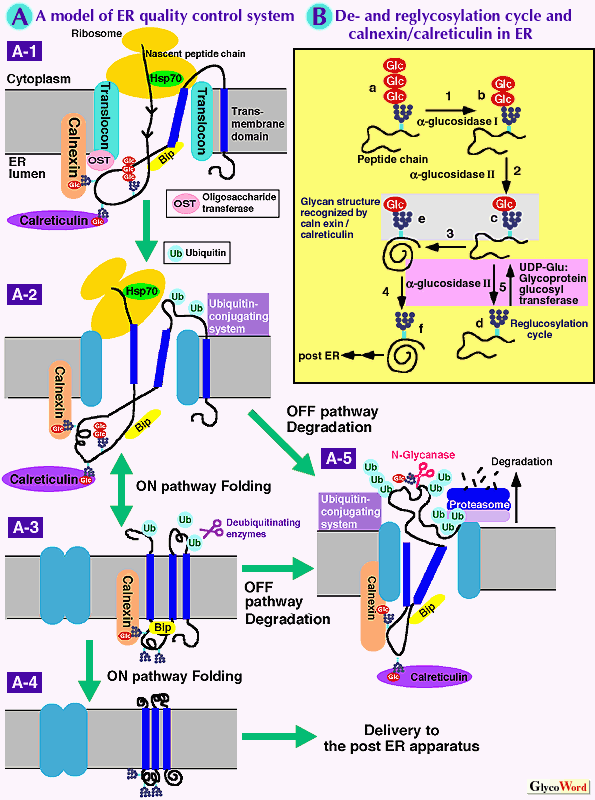
Fig. A A model of ER quality control mechanism (a membrane protein as an example)
A-1: Nascent polypeptides elongating on ribosomes are translocated across the ER membrane through aqueous pores (translocons) and N-linked glycans are transferred to peptides cotranslationally by oligosaccharide tranferase present adjacent to translocons. The processing of N-glycan starts immediately after N-glycosylation (see Fig. B). Nascent peptide chains start to associate with various molecular chaperones.
A-2: ER lectin-based molecular chaperones, calnexine/calreticulin bind to monoglucosylated N-glycans on un/misfolded peptide chains. Association and dissociation with chaperones occur and promote folding of peptides. Some domains of un/misfolded nascent peptide chains can be recognized and modified with ubiquitin by the ubiquitin conjugating system.
A-3: After completion of peptide synthesis, proteins leave translocons, and the folding process continues until peptides attain their native conformations.
A-4: When proteins acquire tertiary and quaternary structures, the mature proteins leave the ER and are delivered to the Golgi apparatus.
A-5: When newly synthesized proteins fail to fold correctly, ER quality control machinery transports the mis/unfolded proteins to the outside of the ER through pores on the ER membrane with the aid of ER chaperone, Bip and cytoplasmic ubiquitin/proteasome system. The exiled peptides are de-glycosylated by N-glycanase, and modified with ubiquitin moieties, subsequently degraded by proteasome.
Fig. B Lectin-like molecular chaperones, calnexin and calreticulin and re- and de-glucosylation cycle.
B-1: alpha-Glucosidase I cotranslationally cleaves one glucose moiety (b) from original high mannose type N-glycans (a) on peptides.
B-2: alpha-Glucosidase II further cleaves the second mannose residue, arising monoglucosyl N-glycans (c), which are recognized by calnexin and calreticulin.
B-3: Peptides attain the native structure (e).
B-4: alpha-Glucosidase II removes the third glucose from folded proteins (f). As calnexin and calreticulin do not have the affinity with this N-glycan, the mature proteins are dissociated from lectins. Then the released proteins are delivered to the post ER compartment.
B-5: alpha-Glucosidase II cleaves the third glucose (d), and peptides are dissociated from calnexin/calreticulin. UDP-Glucose: glycoprotein glucosyltransferase recognizes the non-native peptides and reglucosylate the N-glycans on the peptides(c). Consequently, the non-native peptides are reassociated with calnexin/calreticulin. This on- and off-cycle is repeated until proteins either attain the native structure or are deported from the ER. |
|
|
| Sachiko Sato (Laval University, CHUL Research Center) | |
| | |
|
| References | (1) | RR, Kopito ; ER quality control : The cytoplasmic connection, Cell 88 , 427-430, 1997 |
| (2) | ES, Trombetta, A, Helenius : Lectins as chaperones in glycoprotein folding, Current Opinion in Structural Biology 8, 587-592,1998 |
| (3) | S,Sato, CL, Ward, RR, Kopito : Cotranslational ubiquitination of cystic fibrosis transmembrane conductance regulator in vitro, J. Biol. Chem. 273, 7189-7192, 1998 |
| (4) | I, Wada, M, Kai, S,Imai, F, Sakane, H, Kanoh : Promotion of tranferrin folding by cyclic interactions with calnexin and calreticulin, EMBO J. 16, 5420-5432, 1997 |
| (5) | S, Sato, CL, Ward, M, Krouse, JJ, Wine, RR, Kopito : Glycerol reverses the folding phenotype of the most common cystic fibrosis mutation. J. Biol. Chem. 271, 635-638, 1996 |
| | |
| |
| Mar.15, 1999 |
|
| |
|
|
|
|



