|
Transglycosylation Mechanism of Endoglycanases from the Viewpoint of Oligosaccharide Synthesis
|
|
|
 |
Glycosyl hydrolases can be classified into glycosidases and endoglycanases according to their mode of action toward glycosidic compounds. Glycosidases hydrolyze the glycosidic bond of small glycosyl compounds, whereas glycanases cleave the internal glycosidic bonds of polysaccharides. These enzymes catalyze not only hydrolysis but also transglycosylation reaction. Glycosidases have a pocket in their catalytic sites (Fig. 1A). Endoglycanases have a cleft where a polysaccharide substrate is strictly recognized through hydrogen bonds (Fig. 1B). Due to the strict recognition, transglycosylation proceeds in a highly regio- and stereoselective manner. This article deals with the mechanism of hydrolysis and transglycosylation catalyzed by endoglycanases from the viewpoint of oligosaccharide synthesis. |
|
|
|
|
|
|
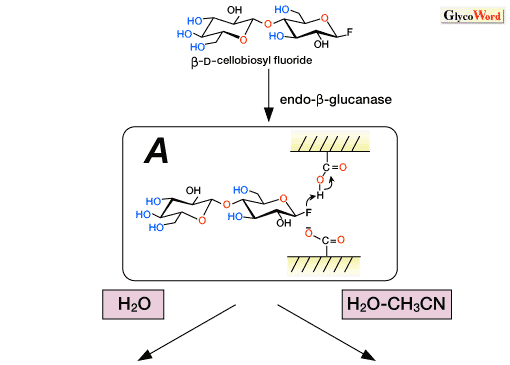
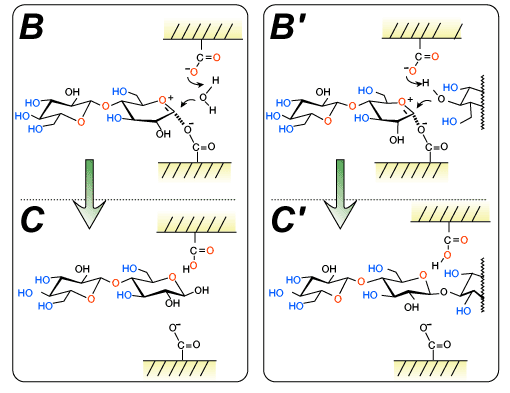
Various glycosyl donors have been developed for enzymatic transglycosylations. Among them, glycosyl fluoride, a sugar derivative whose anomeric hydroxyl group is replaced by a fluorine atom, is one of the most frequently employed substrates. At the first stage of the transglycosylation, the fluorine atom is protonated by a carboxylic acid in the catalytic site to give an oxocarbenium ion intermediate (Fig. 2A-B').
At the second stage, water and the hydroxyl group of a glycosyl acceptor attack the resulting intermediate, giving rise to the hydrolyzate and an oligosaccharide, respectively (Fig. 2C').
The endo-ß-glucanase-catalyzed synthesis of cellooligosaccharides has been demonstrated in an aqueous organic media using ß-cellobiosyl fluoride as the glycosyl donor. As an extension of this reaction, various enzymatic polycondensation reactions of modified cellobiosyl fluorides were demonstrated with the aim of synthesizing functional cellulose derivatives.
|
|
|
| Fig. 2 |
 |
 |
|
|
|
|
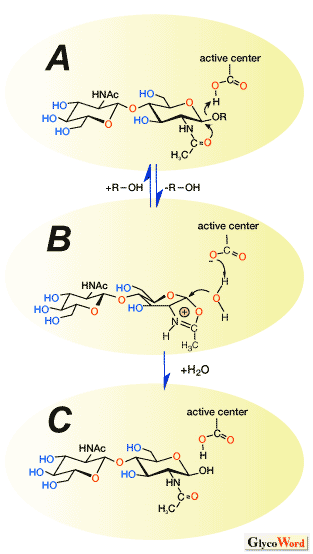
Chitinase, the hydrolytic enzyme of chitin, has also been utilized for efficient glycosylation reactions. Chitinase can be classified into two families, 18 and 19, based on amino acid sequence similarity. Recently, a novel mechanism that involves an oxazolinium ion intermediate has been proposed. According to this mechanism the glycosidic bond is cleaved as follows. First, the oxygen of the glycosidic bond is protonated by the carboxylic acid of an acidic amino acid followed by the formation of the oxazolinium ion intermediate by the nucleophilic attack of the amide carbonyl group to the anomeric center (Fig. 3A-B). The resulting oxazolinium ion intermediate is then attacked by water to give the hydrolyzate (Fig. 3B-C).
Regio- and stereoselective addition reactions of various glycosyl acceptors to sugar oxazoline derivatives have been achieved by using a chitinase under basic reaction conditions, giving rise to the corresponding oligosaccharides. The addition reactions were efficiently promoted and the hydrolysis of the product oligosaccharides does not occur, since the chitinase shows very low hydrolyzing activity under basic conditions. It should be noted that the usage of the sugar oxazoline as a transition state analogue substrate allows the reaction to proceed only in the direction of the addition reaction while suppressing hydrolysis of the product in aqueous media. The present methodology paves the way for the development of enzymatic glycosylation of 2-acetamido-2-deoxysugars in glycotechnolgy.
|
|
|
|
| Fig. 3 |
 |
|
|
|
|
Shin-ichiro Shoda and Masaya Fujita (Graduate School of Engineering, Tohoku University) |
|
|
|
|
|
| References |
(1) |
Shoda S, Fujita M, Kobayashi S : Trends Glycosci. Glycotechnol. 10, 279, 1998
|
|
(2) |
Nishizawa K, Hashimoto K : "The Carbohydrates Chemistry and Biotechnology", Academic Press, Vol. 2A, (1980), p.241.
|
|
(3) |
Tews I, Terwisscha van Scheltinga AC, Perrakis A, Wilson KS, Dijkstra BW, J. Am. Chem. Soc. 119, 7954, 1997
|
|
(4) |
Shoda S, Kiyosada T, Mori H, Kobayashi S, Heterocycles, 52, 599, 2000
|
|
|
|
|
|
| Jun. 15, 2001 |
|
|
|
|
|
|
|



