 |
Although peptides can be easily synthesized by a chemical method, it is almost impossible to synthesize glycopeptides chemically not only because the chemical synthesis of oligosaccharide involves complicated steps, but also because of the difficulty in binding it with the peptide. A practical method of synthesizing a glycopeptide would be to combine the chemical synthesis of glycosyl peptide with the enzymatic method of transglycosylation of endoglycosidase.
Endo-ß-N-acetylglucosaminidase (endo-ß-GlcNAc-ase, EC 3.2.1.96) is a unique endoglycosidase that hydrolyzes N, N'-diacetylchitobiosyl linkages (GlcNAc-GlcNAc) in oligosaccharides bound to asparaginyl residues of various glycoproteins and glycopeptides, and leaves one N-acetylglucosamine (GlcNAc) residue on the protein and peptide moieties.
R-GlcNAc-GlcNAc-Asn-Protein (or peptide) + H2O 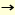 R-GlcNAc + R-GlcNAc +
GlcNAc-Asn-Protein (or peptide) (R: oligosaccharide)
The novel endo-ß-GlcNAc-ases from Mucor hiemalis (Endo-M) and Arthrobacter protophormiae (Endo-A) show transglycosylation activity and can transfer the oligosaccharides from glycoprotein (glycoside donor) to suitable acceptors with a GlcNAc residue during hydrolysis of the glycoprotein (1,2). The transglycosylation activity of endo-ß-GlcNAc-ase catalyzes the following reaction:
R-GlcNAc-GlcNAc-Asn-protein/peptide (glycoside donor) + GlcNAc-Asn-R' (acceptor)
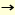 R-GlcNAc-GlcNAc-Asn-R' (transglycosylation R-GlcNAc-GlcNAc-Asn-R' (transglycosylation
product) + GlcNAc-Asn-protein/peptide (R: oligosaccharide, R': peptide)
Then, using the transglycosylation activity of endo-ß-GlcNAc-ase (Endo-M or Endo-A ), glycopeptide is chemo-enzymatically synthesized (3) (Fig.1). |
|
|
The first step in the chemo-enzymatic synthesis of glycopeptide containing N-linked oligosaccharides is the synthesis of glycosylasparagine, which is the building block of N-acetylglucosaminyl peptide. The building block Na-9-fluorenylmethyloxycarbonyl (Fmoc)-Nß-(N-acetylglucosaminyl)-asparagine [Fmoc-Asn(GlcNAc)-OH] is synthesized by reaction of 2-acetamido-3,4,6-tri-O-acetyl-2-deoxy-D-glucopyranosyl azide and Fmoc-aspartic acid alpha-t-butyl ester in the presence of triethylphosphine followed by deacetylation and hydrolysis. The second step is the synthesis of N-acetylglucosaminyl peptide, using Fmoc-Asn (GlcNAc)-OH instead of Fmoc-Asn-OH. The solid-phase synthesis of peptide containing GlcNAc is performed by the dimethylphosphinothioic mixed anhydride (Mpt-MA) method without protecting the hydroxyl groups of the sugar moiety. The third step in the synthesis is the addition of oligosaccharide from a glycosyl donor to N-acetylglucosaminyl peptide using the transglycosylation activity of endo-ß-N-acetylglucosaminidase.
The glycopeptide of calcitonin, which is a calcium-regulating hormone, has been synthesized by the chemo-enzymatic method (4). The glycopeptide of Substance P neuropeptide has also been synthesized by addition of oligosaccharide to the N-acetylglucosaminyl glutamine residue of the peptide. |
|
|
| References |
(1) |
Yamamoto K, Kadowaki S, Watanabe J, Kumagai H, :Transglycosylation activity of Mucor hiemalis endo-ß-N-acetylglucosaminidase which transfers complex oligosaccharides to the N-acetylglucosamine moieties of peptides.Biochem. Biophys. Res. Commun. 203, 244-252, 1994 |
|
(2) |
Takegawa K, Tabuchi M, Yamaguchi S, Kondo A, Kato I, Iwahara S, : Synthesis of neoglycoproteins using oligosaccharide-transfer activity with endo-ß-N-acetylglucosaminidase. J. Biol. Chem. 270, 3094-3099, 1996 |
|
(3) |
Yamamoto K, Fujimori K, Haneda K, Mizuno M, Inazu T, Kumagai H, :Chemo-enzymatic synthesis of a novel glycopeptide using a microbial endoglycosidase. Carbohydr. Res. 305, 415-422, 1998 |
|
(4) |
Mizuno M, Haneda K, Iguchi R, Muramoto I, Kawakami T, Aimoto S, Yamamoto K, Inazu T, : Synthesis of a glycopeptide containing oligosaccharides: chemoenzymatic synthesis of eel calcitonin analogues having natural N-linked oligosaccharides. J. Am. Chem. Soc. 121, 284-290, 1999
|
|
|
|
|
|



