 |
Sialic acids, a family of 9-carbon acidic sugars, show over 50 different molecular species due to various molecular modifications such as acetylation and sulfation. When focused on the substituent at the C-5 position, they are classified into three major types: N-acetylneuraminic acid (Neu5Ac), N-glycolylneuraminic acid (Neu5Gc), and 2-keto-3-deoxy-ᴅ-glycero-ᴅ-galacto-nononic acid (Kdn)(Figure). In mammals, Kdn is present only in trace amounts, thus, Neu5Ac and Neu5Gc are the major sialic acid species, however humans do not synthesize Neu5Gc, which is biosynthesized from its precursor, CMP-Neu5Ac, via a hydroxylation reaction at the CMP-sialic acid level. This reaction in the cytosol involves NADH, cytochrome b5, cytochrome b5 reductase, and CMP-Neu5Ac hydroxylase (CMAH) (1,2). Kozutsumi’s group and Varki’s group independently generated Cmah knockout mice. Neu5Gc was undetectable in both strains, showing that CMAH is essential for Neu5Gc biosynthesis (3,4).
The Neu5Gc/Neu5Ac ratio depends on the expression level of CMAH, which varies among cell types. Therefore, the ratio differs not only among organs within the same species but also among species for the same organ. Moreover, Neu5Gc/Neu5Ac changes within a single cell: In mouse lymphocytes, Neu5Gc is typically the dominant form of sialic acid; however, Cmah expression is suppressed upon activation, leading a decrease in Neu5Gc and a shift toward Neu5Ac dominance (3). Neu5Gc negatively regulates cellular activation in lymphocytes and its reduction upon activation allows lymphocytes to escape this suppression. This suggests the existence of a molecular system that can distinguish a single oxygen atom difference between Neu5Gc and Neu5Ac to regulate cellular activation.
Neu5Gc expression is tightly regulated and significantly different among tissues and cell types. Nevertheless, it shows two notable patterns. One is that Neu5Gc is absent in the brain except for the blood vessels in all animal species examined so far. Although the molecular mechanism underlying this brain-specific Cmah suppression remains unclear, transgenic expression of Neu5Gc in brain neural cells resulted in motor dysfunction caused by impaired myelination, impaired object recognition memory, and increased susceptibility to Neu5Gc-specific pathogens, suggesting that the evolutionary loss of Neu5Gc in the brain may have conferred an advantage (5).
The other characteristic of Neu5Gc expression pattern is the absence in humans. In humans, the CMAH gene contains an Alu insertion, which results in the deletion of a 92-base pair exon, causing a frameshift mutation that renders CMAH a non-functional pseudogene (6). As a result, humans cannot biosynthesize Neu5Gc in the whole body. This mutation seemed to have occurred approximately 2–3 million years ago and represents a definitive difference between humans and our closest relatives, chimpanzees. Various studies using Cmah knockout mice, which lack Neu5Gc, and Cmah transgenic mice, which overexpress Neu5Gc, revealed that this Neu5Gc deficiency allows human-specific diseases/pathological conditions such as typhoid fever, cholera, and muscular dystrophy (5,6,7). Additionally, impaired hearing in older mice and delayed wound healing was reported in Cmah knockout mice (4).
Furthermore, the lack of CMAH in humans is considered to cause human-specific chronic inflammation. Since humans cannot biosynthesize Neu5Gc, Neu5Gc is recognized as a xeno-antigen and immunogenic. In fact, most humans develop anti-Neu5Gc antibodies during infancy. Thus, CMAH-deficient pigs are used in xenotransplantation to avoid immune rejection. On the other hand, human uptake dietary Neu5Gc by eating red meats such as beef and pork. The ingested Neu5Gc is recycled through salvage pathway and incorporated into human glycans, where it is presented as a rare "xeno-autoantigen". This means that xeno-antigen Neu5Gc and anti-Neu5Gc antibodies co-exist in the body, which may induce human-specific chronic inflammation condition triggering atherosclerosis and cancer (7).
It remains uncertain whether the loss of Neu5Gc provided an evolutionary advantage; however, it is clearly involved in host specificity of infectious diseases, and human-specific diseases/pathological conditions. Therefore, the differences in sialic acid composition between human and animal models should be taken into consideration when conducting research on human diseases.
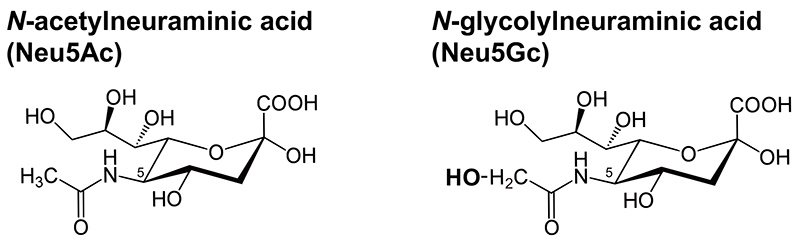
Figure. Structures of Neu5Ac and Neu5Gc
|
|
|---|






