| Ricin-related Protein Family |  |
|
 |
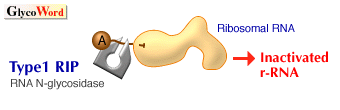
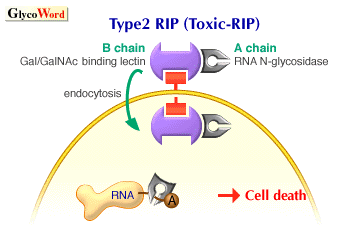
Ricin was found by Stillmark in 1888 as the first plant lectin from the seeds of Ricinus communis. Ricin is a toxic, heterodimeric protein consisting of a Gal/GalNAc-binding subunit, B chain (32 kDa), and a toxic subunit, A chain (ca. 30 kDa), connected through a disulfide linkage. The A chain of ricin has RNA N-glycosidase activity to cleave a specific adenine base from ribosomal RNA, causing the inactivation of the ribosome and inhibition of protein synthesis. The lectin subunit, B chain, of ricin plays an important role of binding to the cell surface glycoconjutgates of target cells and facilitates the internalization and translocation of the toxin to cytosol. Ricin belongs to the group of type 2 ribosome-inactivating proteins (type 2 RIPs), which are distinguished from type 1 RIPs by the presence of the B chain. The latter group, type 1 RIPs, are much less cytotoxic due to the lack of the B chain. However, type 1 RIPs can still be internalized by fluid-phase endocytosis. In the case of saporin obtained from Saponaria officinalis, it was reported that saporin first binds to the alpha2-macroglobulin receptor on human cells and is then internalized to cytosol. Type 1 RIPs have been isolated from over 50 plants both monocots and dicots, indicating a more general occurrence compared with type 2 RIPs. In evolution, it has been proposed that type 2 RIPs were generated by the fusion of ancestral genes encoding a type 1 RIP and a Gal/GalNAc-binding lectin. So far, only six type 2 RIPs with potent toxicity have been reported (ricin, abrin, viscumin, modeccin, volkensin and Eranthis hyemalis lectin).
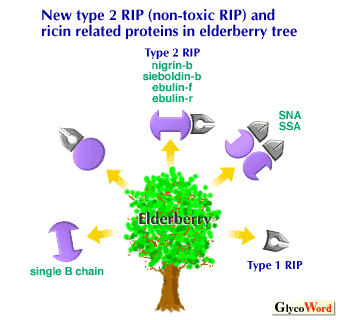
Recently, novel non-toxic type 2 RIPs and ricin-related proteins have been isolated from the bark, leaf and rhizome of elderberry trees belonging to the genus Sambucus. These non-toxic type 2 RIPs, such as nigrin-b, sieboldin-b, ebulin-f and ebulin-r, have structure and RNA N-glycosidase activity similar to those of ricin and other classical type 2 RIPs. These non-toxic type 2 RIPs showed the same binding specificity towards Gal/GalNAc residues as ricin, but a new RIP which was recently isolated was devoid of carbohydrate-binding activity. Interestingly, these type 2 RIPs from elderberry plants showed a potent inhibitory activity against the in vitro protein synthesis of rabbit reticulocyte lysate similar to ricin but showed little toxicity to cells and mice. The tetrameric bark lectins (SNA and SSA) from the bark of these plants have been known to show a unique sugar-binding specificity towards the Neu5Ac-alpha2-6Gal/GalNAc unit. Recent cDNA cloning of these lectins showed that both A and B subunits of these lectins have homologus primary as well as tertiary structures with A (toxic subunit) and B (Gal/GalNAc-specific lectin subunit) chains of ricin. However, these lectins showed little toxicity to cells and mice or inhibitory activity against the in vitro protein synthesis of rabbit reticulocyte lysate. These characteristics were quite different from those of a structurally related tetrameric lectin, RCA-120, which strongly inhibits the protein synthesis of rabbit reticulocyte lysate. In addition to these type 2 RIPs and related proteins, a type 1 RIP (without B chain) and a free B chain-like lectin (without A chain) were isolated from these plants.
Some type 1 RIPs have been reported to possess antiviral or antifungal activity. The A chains of type 1 and type 2 RIPs have been used as the toxic component of immunotoxins. Various type 1 and 2 RIPs and other ricin-related proteins found in elderberry plants raise new and interesting questions regarding the biological functions as well as the evolutional relationships of ricin-related proteins. | |
|
Figure Legend
Structure and mechanism of toxicity of Type I and Type II ribosome-inactivating proteins and several ricin related proteins in elderberry tree.
 |  |
 |
 |
|
|
| Hanae Kaku (National Institute of Agrobiological Resources) | |
|
|
| References | (1) | Lord, J M et al: Ricin:structure, mode of action, and some curret applications. FASEB J. 8 , 201-208, 1994 |
| (2) | Hartley, M R et al: The structure and function of ribosome-inactivating proteins. Trends Plant Sci, 1, 254-259, 1996 |
| (3) | Van Damme, E J M et al: Isolation and molecular cloning of a novel type 2 ribosome-inactivating protein with an inactive B chain from elderberry (Sambucus nigra) bark. J. Biol. Chem. 272, 8353-8360, 1997 |
| (4) | Citores, L et al: Characterization of a new non-toxic two-chain ribosome-inactivating protein and a structurally-related lectin from rhizome of dwarf elder (Sambucus ebulus L.). Cell. Mol. Biol, 43, 485-499, 1997 |
| (5) | Rojo, M A et al: Isolation, cDNA cloning, biological properties, and carbohydrate binding specificity of sieboldin-b, a type II ribosome-inactivating protein from the bark of Japanese elderberry (Sambucus sieboldiana). Arch. Biochem. Biophys, 340, 185-194, 1997 |
| (6) | Kaku, H et al: Sialylated oligosaccharide-specific plant lectin from Japanese elderberry (Sambucus sieboldiana) bark tissue has homologus structure to type II ribosome-inactivating proteins, ricin and abrin. J. Biol. Chem, 271, 1480-1485,1996 |
| | |
| |
| Mar.15, 1998 |
|
| |
|
|
|
|



