 |
Pholiota squarrosa lectin (PhoSL) is a chemically synthesizable 40-amino-acid mini-lectin, which specifically recognizes “core fucosylation” at the innermost N-acetylglucosamine (GlcNAc) of N-glycans (Fig. 1A) (1). The PhoSL gene actually codes for a 180-amino-acid protein that harbors three repeats of 40-amino-acid similar motifs (30–34 amino acids are identical among the three motifs) (2). The PhoSL sequence shown in ref. 1 is likely due to the analysis of mixture of the three motifs after proteolytic cleavage of the linker sequences. It was shown that this peptide and those corresponding to the three motifs in the gene showed similar activities. PhoSL forms a symmetrical trimer with a “β-prism” structure (Fig. 1B) (3, 4). According to the PhoSL gene, the natural PhoSL should form a heterotrimer consisting of the three similar peptides, although that produced by chemical synthesis or recombinant expression of either of the motifs should be a homotrimer.
Core-fucosylated glycans are recognized at the sugar-binding pocket formed between the polypeptide chains in the trimer (Fig. 1B,C). The trisaccharide fucose(α1–6)[GlcNAc(β1–4)]GlcNAc that specifies the core fucose structure enters the pocket with the methyl group of fucose and the acetyl group of the second GlcNAc pointing to the bottom. The strong affinity is obtained by hydrogen bonds and hydrophobic interactions formed within the pocket. Glycans have their intrinsically stable conformations, which are observed, e.g., by molecular dynamics simulations. The trisaccharide bound to PhoSL was found to adopt such an intrinsically stable conformation (5). In contrast, the other types of fucosylated glycans, i.e., the H-type glycans possessing the fucose(α1–2) linkage and the Lewis-type glycans possessing the fucose(α1–3) or fucose(α1–4) linkage, would cause inevitable steric hindrance with amino acids (e.g., Tyr23 and Trp28) forming the sugar-binding pocket of PhoSL, when we try to dock them within their stable conformations (5). This well explains the exclusive specificity of PhoSL to the core-fucosylated glycans.
Because fucosylation is known to be related to cancers and the precancerous lesions, PhoSL is expected to be used in diagnosis. For example, haptoglobin in the serum from patients of pancreatic cancer and chronic pancreatitis possess fucosylated N-glycans (6). A combination of PhoSL and Aleuria aurantia lectin (AAL) that also recognizes other types of fucosylation enables detailed analyses, where it was shown that the ratio of core fucosylation is lower in pancreatic cancer than in pancreatitis. Researches using PhoSL toward more precise diagnosis are also conducted for prostate cancer (7).
Many viruses possess core-fucosylated N-glycans on the envelope. For example, the spike protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) possesses core-fucosylated N-glycans. It was shown that PhoSL strongly binds to the spike protein and interferes with the SARS-CoV-2 infection (8). Because the glycosylation sites are not affected by most of the virus mutations, the effect of PhoSL is not affected either, and similarly inhibits the infection by the ancestral Wuhan and Omicron-variant strains.
When PhoSL binds to core fucosylation site at the innermost GlcNAc, it can also interact with amino acids of the protein to which the glycan is attached (8). It would be thus possible to confer protein specificities by appropriate mutations of PhoSL. Chemical synthesis of PhoSL enables incorporation of a variety of unnatural amino acids. Therefore, PhoSL is likely to be a potential material to develop therapeutic or diagnostic agents.
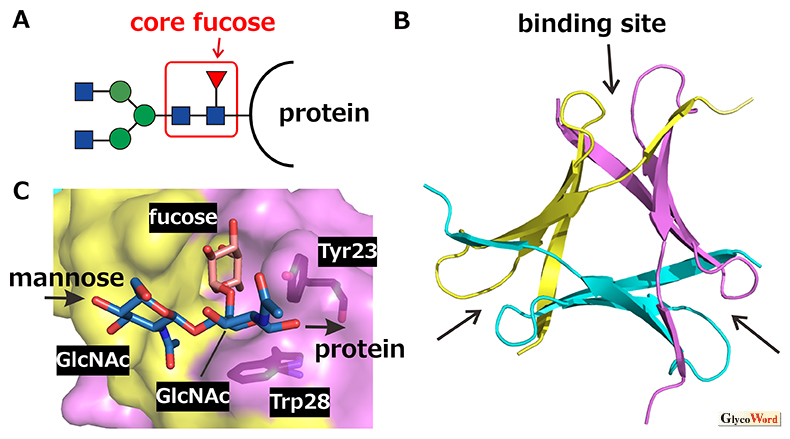
Fig. 1
Trimerization of PhoSL and recognition of core-fucosylated N-glycans. A. Core-fucosylated N-glycan (red: fucose, blue: GlcNAc, green: mannose). B. Three-dimensional structure of PhoSL trimer. Respective monomers are differently colored. Sugar-binding pockets are indicated by arrows. C. Recognition of core-fucose trisaccharide in the binding pocket. The molecular graphics are generated by PyMOL ver. 2.5 based on PDB entry 7VU9.
Kazuhiko Yamasaki
(Biomedical Research Institute, Advanced Industrial Science and Technology (AIST))
| References |
| (1) |
Kobayashi Y, Tateno H, Dohra H, Moriwaki K, Miyoshi E, Hirabayashi J, Kawagishi H: A novel core fucose-specific lectin from the mushroom Pholiota squarrosa. J. Biol. Chem. 287, 33973-82, 2012 |
| (2) |
Kobayashi Y, Mizuno T, Matsui H, Hirabayashi J, Tateno H, Kawagishi H, Dohra H: Jpn Kokai Tokkyo Koho, JP2011-148735, 2011 |
| (3) |
Yamasaki K, Yamasaki T, Tateno H: The trimeric solution structure and fucose-binding mechanism of the core fucosylation-specific lectin PhoSL. Sci. Rep. 8, 7740, 2018 |
| (4) |
Cabanettes A, Perkams L, Spies C, Unverzagt C, Varrot A: Recognition of complex core fucosylated N-glycans by a mini lectin. Angew. Chem. Int. Ed. Engl. 130, 10335-10338, 2018 |
| (5) |
Yamasaki K, Kubota T, Yamasaki T, Nagashima I, Shimizu H, Terada RI, Nishigami H, Kang J, Tateno M, Tateno H: Structural basis for specific recognition of core fucosylation in N-glycans by Pholiota squarrosa lectin (PhoSL). Glycobiology 29, 576-587, 2019 |
| (6) |
Miyoshi E, Kamada Y:Application of glycoscience to the early detection of pancreatic cancer. Cancer Sci. 107, 1357-1362, 2016 |
| (7) |
Llop E, Ferrer-Batallé M, Barrabés S, Guerrero PE, Ramírez M, Saldova R, Rudd PM, Aleixandre RN, Comet J, de Llorens R, Peracaula R: Improvement of prostate cancer diagnosis by detecting PSA glycosylation-specific changes. Theranostics 6, 1190-1204, 2016 |
| (8) |
Yamasaki K, Adachi N, Ngwe Tun MM, Ikeda A, Moriya T, Kawasaki M, Yamasaki T, Kubota T, Nagashima I, Shimizu H, Tateno H, Morita K: Core fucose-specific Pholiota squarrosa lectin (PhoSL) as a potent broad-spectrum inhibitor of SARS-CoV-2 infection. FEBS J. 290, 412-427, 2023 |
Jun. 15, 2023
|
|---|







