 |
C-type lectin is one of the most ubiquitous lectins and conserved from Archaea to human. C-type lectin receptor is a kind of C-type lectin family members and is type II transmembrane protein harboring sugar binding domain named carbohydrate recognition domain (CRD) in the extracellular side. C-type lectin receptors transduce signals via a characteristic intracellular signaling motifs upon ligand binding to extracellular CRDs. C-type lectin receptors expressed on macrophages and dendritic cells recognize glycans and glycolipids derived from foreign pathogens or damaged self and initiate immune responses such as phagocytosis and production of inflammatory cytokines.
Mincle (Macrophage-inducible C-type lectin, gene symbol: Clec4e) is one of the immune activation C-type lectin receptors and expresses on monocytes and macrophages (1). When Mincle is activated by ligand stimulation, Mincle activates adaptor molecule, FcRγ, via its immunoreceptor tyrosine-based activation motif (ITAM) and recruits tyrosine kinase Syk via ITAM. This signal further transduces downstream and activates several transcription factors such as NF-κB and produces various inflammatory cytokines like TNF and IL-6. Mincle can recognize various exogenous and endogenous (glyco)lipid ligands.
As for exogenous (non-self) Mincle ligands, trehalose 6,6’-dimycholate (TDM), an abundant glycolipid in cell wall component of Mycobacterium tuberculosis, is one of the best characterized exogenous ligands for Mincle. TDM, also called cord factor, stimulates Mincle leading to the production of inflammatory cytokines and nitric oxide (2). Also, TDM stimulation is involved in the formation of lung granuloma. Previous structural studies have shown that Mincle precisely recognizes trehalose disaccharide (Glcα1-1Glc), the sugar moiety of TDM, via CRD (Figure 1). One glucose residue of trehalose disaccharide interacts with CRD via calcium ion, while the other glucose residue tightly interacting with amino acid residues around the calcium ion binding site. Interestingly, a characteristic groove composed of hydrophobic amino acids was found around the trehalose binding site, and it is presumed that the acyl chains of TDM are recognized via this groove. Human Mincle also recognizes glycerol monomycolate (GroMM), a mycolic acid-containing lipid present in the cell wall of mycobacteria, in a species-specific manner (4). Mutagenesis revealed that this species-specific interaction is due to differences in the amino acid residues in the hydrophobic groove. Because GroMM has no sugar moiety, the binding mode to Mincle is unknown and awaits further elucidation.
Recently another exogenous ligand of Mincle was found in Helicobacter pylori. Mincle recognizes Cholesteryl acyl α-glucoside (αCAG), a metabolite of H. pylori, and causes exacerbation of gastritis (5). Interestingly, H. pylori is unable to synthesize cholesterol on its own, thus it takes up cholesterol from the host and synthesizes αCAG.
As for endogenous (self) ligands, Mincle recognizes various (glyco)lipids released from damaged self and triggers immune responses such as inflammation. One such Mincle ligand is β-glucosylceramide (βGlcCer) (6). βGlcCer is normally found inside of the cells, such as the endoplasmic reticulum and Golgi membranes, thus βGlcCer in normal cells does not contact with Mincle. However, when cells are damaged, βGlcCer is released to the outside, stimulating Mincle and inducing the production of inflammatory cytokines. This immune response does not occur with lactosylceramide or galactosylceramide, suggesting that sole glucose is important for Mincle recognition. Another endogenous ligand is cholesterol crystals, which are involved in various chronic inflammations (7). Human Mincle, but not mouse Mincle, has been shown to recognize cholesterol crystals and induce the production of inflammatory cytokines such as TNF and MIP-2. This interaction is also species-specific and mutant analysis suggests that the binding site is different from that of TDM, though the precise recognition mode of cholesterol crystals by Mincle has been still unclarified.
Mincle widely accepts various (glyco)lipid ligands derived from both exogenous and endogenous origins and promotes immune responses against infection and inflammatory diseases. Several Mincle ligands show species-specific manners, reflecting that these ligands may contribute to the species-specific immune response to the environment. Compared to these physiological analyses, structural studies on ligand recognition in Mincle have been limited. Further progress in multidimensional analysis from molecular recognition to physiological function is expected in the future.
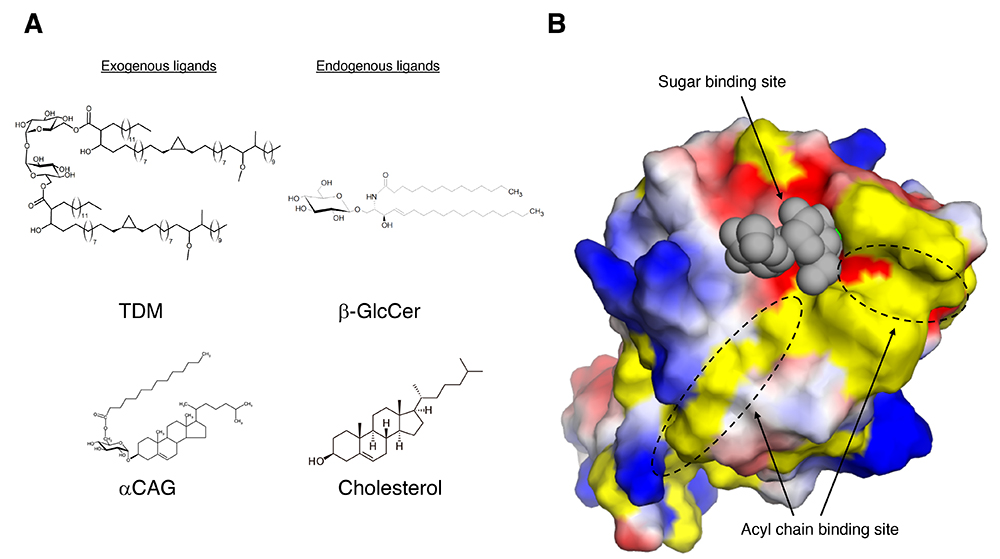
Figure 1. Examples of glycolipid ligands of Mincle and ligand recognition mechanism of Mincle.
A. Exogenous and endogenous Mincle ligands described in this review. TDM: Trehalose 6,6'-dimycholate, CAG: Cholesteryl acyl α-glucoside, β-GlcCer: β-glucosylceramide. B. 3D structure of Mincle complexed with trehalose, sugar moiety of TDM (PDB code: 4ZRW). Mincle CRD and trehalose are shown as surface and sphere models, respectively. The surface color of Mincle CRD indicates charged (positive charge: blue, negative charge: red) and hydrophobicity (yellow). In the Figure, they are written as Sugar binding site and Acyl chain binding site, respectively. This figure was created using PyMOL 2.0.
|
Masamichi Nagae, Sho Yamasaki
(Research Institute for Microbial Diseases and Immunology Frontier Research Center, Osaka University)
| References |
| (1) |
Matsumoto M, Tanaka T, Kaisho T, Sanjo H, Copeland NG, Gilbert DJ, Jenkins NA, Akira S: A novel LPS-inducible C-type lectin is a transcriptional target of NF-IL6 in macrophages. J. Immunol. 163, 5039-5048, 1999 |
| (2) |
Ishikawa E, Ishikawa T, Morita YS, Toyonaga K, Yamada H, Takeuchi O, Kinoshita T, Akira S, Yoshikai Y, Yamasaki S: Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J. Exp. Med. 206, 2879-2888, 2009 |
| (3) |
Feinberg H, Jégouzo SA, Rowntree TJ, Guan Y, Brash MA, Taylor ME, Weis WI, Drickamer K: Mechanism for recognition of an unusual mycobacterial glycolipid by the macrophage receptor mincle. J. Biol. Chem. 288, 28457-28465, 2013 |
| (4) |
Hattori Y, Morita D, Fujiwara N, Mori D, Nakamura T, Harashima H, Yamasaki S, Sugita M: Glycerol monomycolate is a novel ligand for the human, but not mouse macrophage inducible C-type lectin, Mincle. J. Biol. Chem. 289, 15405-15412, 2014 |
| (5) |
Takato K, Ishida H, Nagai S, Illarionov P, Stocker BL, Timmer MSM, Smith DGM, Williams SJ, Bamba T, Miyamoto T, Arita M, Appelmelk BJ, Yamasaki S: Helicobacter pylori metabolites exacerbate gastritis through C-type lectin receptors. J. Exp. Med. 218, e20200815, 2021 |
| (6) |
Nagata M, Izumi Y, Ishikawa E, Kiyotake R, Doi R, Iwai S, Omahdi Z, Yamaji T, Miyamoto T, Bamba T, Yamasaki S: Intracellular metabolite β-glucosylceramide is an endogenous Mincle ligand possessing immunostimulatory activity. Proc. Natl. Acad. Sci. U S A 114, E3285-E3294, 2017 |
| (7) |
Kiyotake R, Oh-Hora M, Ishikawa E, Miyamoto T, Ishibashi T, Yamasaki S: Human Mincle Binds to Cholesterol Crystals and Triggers Innate Immune Responses. J. Biol. Chem. 290, 25322-25332, 2015 |
Jun. 15, 2023
|
|---|







