|
The Concept of an alpha-Amylase Family
|
 |
|
 |
Starch is a mixture of amylose which is composed of glucose polymer with essentially only alpha-1,4 linkages, and amylopectin, which is composed of alpha-1,4-linked glucose polymer branched by alpha-1,6 linkages. The enzymes which specifically catalyze the hydrolysis or synthesis of the glucosidic linkages of starch are represented by four types as follows:
| 1. |
hydrolysis of alpha-1,4-glucosidic linkages; alpha-amylase (EC 3.2.1.1) |
| 2. |
hydrolysis of alpha-1,6-glucosidic linkages; pullulanase (EC 3.2.1.41) or isoamylase (EC 3.2.1.68) |
| 3. |
transglycosylation to form alpha-1,4-glucosidic linkages; cyclodextrin glucanotransferase (CGTase, EC 2.4.1.19) |
| 4. |
transglycosylation to form alpha-1,6-glucosidic linkages; 1,4-alpha-D-glucan:1,4 -alpha-D-glucan 6-alpha-D-(1,4-alpha-D-glucano)-transferase (branching enzyme, EC 2.4.1.18) |
Each of the four reactions are representatively catalyzed by four individual types of enzymes, described above. However, some exceptions have been reported: alpha-amylases weekly catalyze alpha-1,4 transglycosylation in addition to the main reaction, alpha-1,4 hydrolysis. CGTases feebly catalyze alpha-1,4 hydrolysis in addition to the main reaction, alpha-1,4 transglycosylation. We can, therefore, reasonably conclude that the boundary between "glucanohydrolases" and "glucanotransferases" is not always clear. Furthermore, some alpha-amylases have been reported to catalyze alpha-1,6 hydrolysis. Some pullulanases from thermophile have recently been reported to hydrolyze not only alpha-1,6- but also alpha-1,4-glucosidic linkages. Thus, we know the existence of enzymes on the boundary between alpha-amylase and pullulanase/isoamylase which have been classified as enzymes specific to apha-1,4- and alpha-1,6-glucosidic linkages, respectively. Nevertheless, these facts have not attracted much attention and are considered to be minor exceptions or trivial side reactions.
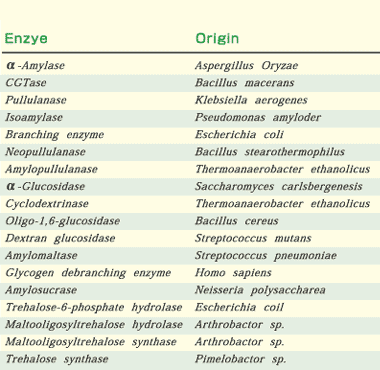
Many primary sequences of amylases and related enzymes from various origins have been reported since the 1980’s. The rapid increase of in the number of available sequences has made it possible to compare sequences. The existence of four highly conserved regions was clearly pointed out in alpha-amylases, CGTases, isoamylases, pullulanases, branching enzymes, and neopullulanases (EC 3.2.1.135)(Fig.1). The three-dimensional structures of alpha-amylases, CGTases, isoamylase, and neopullulanase are based on (beta/alpha)8-barrel as their main domains.
|
|
|
|
|
 |
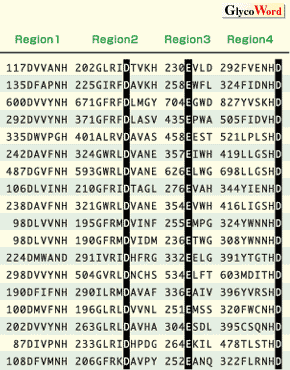 |
| Fig. 1. Enzymes belonging to the alpha-amylase family and the four highly conserved regions. Three invariable catalytic sites are indicated by open circles. Numbering of the amino acid sequences of the enzymes start at the amino-terminal amino acid of each mature enzyme. |
|
|
|
|
|
As described above, the hypothesis that most of amylases and their related enzymes posses similar structure leaves no room for doubt. However, we thought that the structural similarity was not sufficient to gather these enzyme together into one family and that we should clarify the common catalytic mechanisms of them. Because, (beta/alpha)8-barrel structure was first reported for triose phosphate isomerase, and was therefore called TIM-barrel. Triose phosphate isomerase have no relation to amylases and related enzymes with respect of its action pattern and function. Thus, there exist lots of enzymes which do not relate to their function at all, but have similar structures. Based on the background described above, it was proved that the four reactions, alpha-1,4 and alpha-1,6 hydrolysis and alpha-1,4 and alpha-1,6 transglycosylation, could be catalyzed by the same mechanism based on the experimental results using neopullulanase (1). Indeed, one active center of neopullulanase participated in all the four reactions (2).
Based on the structural similarity and common catalytic mechanisms among most of amylases and related enzymes, a general idea for one enzyme family, the alpha-amylase family, was consequently proposed (3). The alpha-amylase family was defined for enzymes that satisfy the following four requirements:
| i) |
They act on alpha-glucosidic linkages. |
| ii) |
They hydrolyze alpha-glucosidic linkages to produce alpha-anomeric mono- and oligo-saccharides or form alpha-glucosidic linkages by transglycosylation. |
| iii) |
They have four highly conserved regions in their primary sequence containing all catalytic and most of the important substrate-binding sites. |
| iv) |
They have Asp, Glu, and Asp residues as catalytic sites corresponding to Asp-206, Glu-230, and Asp-297 of Taka-amylase A. |
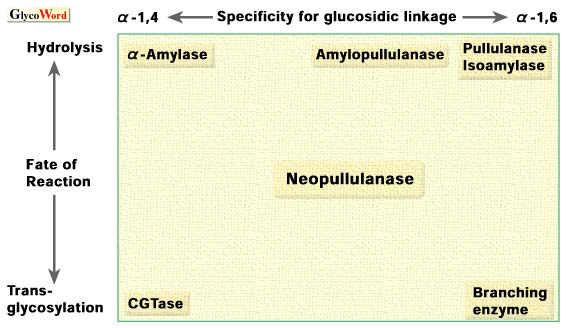
In order to explain the concept of an alpha-amylase family, the relation of specificities for the target linkage and reaction of the enzymes typically belong to the alpha-amylase family is schematically represented in Fig. 2 (4). |
|
|
|
Fig. 2. Schematic representation of the relationship of enzymes that belong to the alpha-amylase family according to type of reaction.
|
|
|
|
|
|
Takashi Kuriki (Biochemical Research Laboratory, Ezaki Glico Co., Ltd.) |
|
|
|
|
|
| References |
(1) |
Kuriki T, Takata H, Okada S, Imanaka T : Analysis of active center of Bacillus stearothermophilus neopullulanase. J.Bacteriol.173,6147-6152, 1991 |
|
(2) |
Kuriki T, Kaneko H, Yanase M, Takata H, Shimada J, Handa S, Takada T, Umeyama H, Okada S : Controlling substrate preference and transglycosylation activity of neopullulanase by manipulating steric constraint and hydrophobicity in active center. J. Biol. Chem. 271,17321- 17329, 1996 |
|
(3) |
Takata H, Kuriki T, Okada S, Takesada Y, Iizuka M, Minamiura N, Imanaka T : Action of neopullulanase: neopullulanase catalyzes both hydrolysis and transglycosylation at alpha-(1 4) - and alpha-(1 4) - and alpha-(1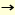 6) - glucosidic linkages. J. Biol. Chem. 267,18447-18452,1992 6) - glucosidic linkages. J. Biol. Chem. 267,18447-18452,1992 |
|
(4) |
Kuriki T, Imanaka T, The concept of the alpha-amylase family: structural similarity and common catalytic mechanism. J. Biosci. Bioeng. 87, 557-565, 1999 |
|
|
|
|
|
| Sep. 15, 2000 |
|
|
|
|
|
|
|



