

 |
 |
Solution Structure of Heparin-binding Growth Factor Midkine - Interaction with Heparin |
 |
|||||||||||||||||||
 |
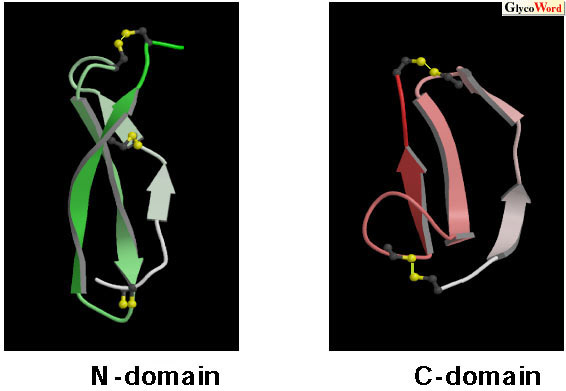
Upon the signal transduction of growth factors, growth factors bind to the extracellular domain of receptors, causing the oligomerization of receptors and intermolecular autophosphorylation of tyrosine residues that result in stimulation of protein tyrosine kinase activity. Therefore, receptor oligomerization is the key step for the signal transduction of growth factors. In the case of heparin-binding growth factors, heparin sulfate proteoglycans are required for the oligomerization of ligands and/or the presentation of these oligomers to their appropriate signaling receptors. Midkine (MK) is a heparin-binding growth factor discovered by Muramatsu et al, (refer to review 1). MK enhances neurite outgrowth, neuronal cell survival and plasminogen activator activity. Strong expression of MK is found in various human tumors. Therefore, MK is expected to be a tumor marker and target for tumor therapy. The amino acid sequence of MK is unrelated to other proteins and forms a new growth factor family with pleiotrophin, which was discovered after MK. MK is structurally divided into two domains. We determined the solution structures of the two domains by NMR (2): MK residues 1-59 (N-domain) and MK residues 62-104 (C-domain). Both domains have anti-parallel |
|||||||||||||||||||
 |
||||||||||||||||||||
| Fig. 1 Solution structures of the N-domain and C-domain (2). Main chain folds of each peptide from the N- to C-terminus are represented by gradation from white to green or pink. The disulfide bonds are shown in yellow. |
||||||||||||||||||||
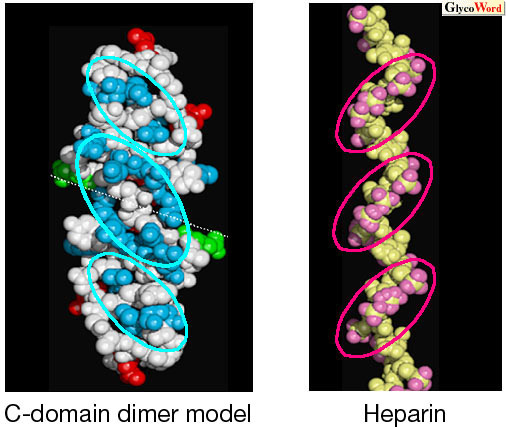
| It has been reported that some MK activity requires dimerization of MK. The binding of MK to heparin of more than 20 monosaccharide units induced MK dimerization (2). These results suggest that the MK dimer is the active form, and that heparan sulfate is required for the dimerization of MK. We proposed a molecular model for the dimer formation of MK on heparin oligosaccharides. If C-domains form a head-to-head dimer, the shape and the location of the basic clusters and those of the sulfate clusters on heparin oligosaccharide fit well with each other (Fig. 2). This feature implies that the MK dimer binds heparin more strongly than the monomer. |
||||||||||||||||||||
 |
||||||||||||||||||||
| Fig. 2 The model for the C-domain dimer (blue, basic residues; red, acidic residues; green, Gln) and heparin (pink, oxygens of sulfate groups). Positively charged clusters (blue circles) of the C-domain fit to negatively charged clusters (pink circles) of heparin. |
||||||||||||||||||||
|
MK receptors are thought to be proteoglycans such as protein-tyrosine phosphatase |
||||||||||||||||||||
| Wakana Iwasaki (RIKEN Harima Institute) Fuyuhiko Inagaki (Graduate School of Pharmaceutical Sciences, Hokkaido University) |
||||||||||||||||||||
|
||||||||||||||||||||
| Sep. 30, 2004 | ||||||||||||||||||||
|
|
||||||||||||||||||||
|
||||||||||||||||||||