Pathogenesis and therapeutic strategy of glycosylation-defective muscular dystrophy (dystroglycanopathy) | ||||||||||||||
 |
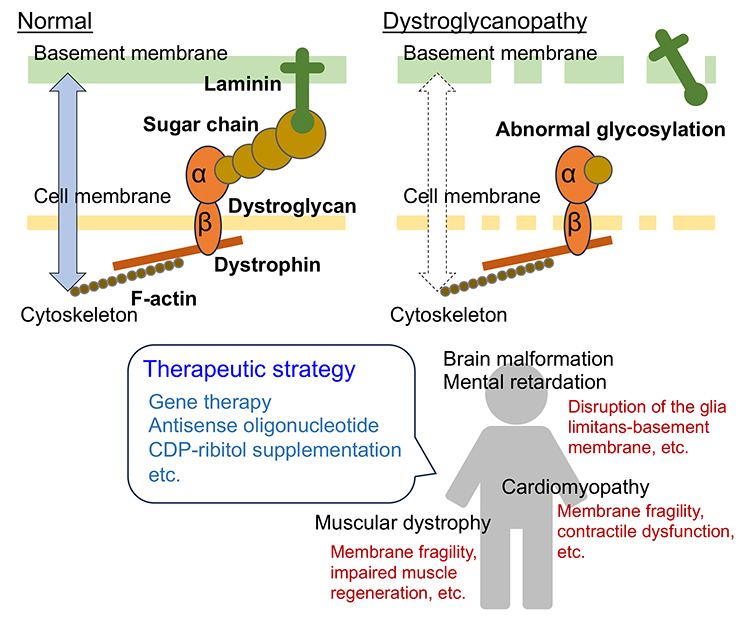
Muscular dystrophy is a group of inherited disorders characterized by progressive muscle weakness with myofiber degeneration and regeneration. A sub-group of muscular dystrophies called dystroglycanopathy (DGpathy) is caused by abnormal glycosylation of dystroglycan (DG), a receptor for laminin in the basement membrane. In Japan, the predominant DGpathy is Fukuyama-type muscular dystrophy (FCMD) that is caused by mutations in the fukutin (FKTN) gene. DG consists of α- and β-chains. α-DG extracellularly binds to laminin. The transmembrane β-DG anchors α-DG on the surface of the cell membrane and binds intracellularly to the actin-binding protein, dystrophin. Abnormal glycosylation of α-DG disrupts the laminin (basement membrane)-DG-dystrophin-actin (cytoskeleton) linkage, weakening muscle fibers and causing disease (Figure 1). The essential sugar chain structure with laminin-binding activity is a repeating unit of the disaccharides xylose and glucuronic acid, and is now called matriglycan (see “Structure and biosynthesis of O-mannosyl glycans in mammals” for details). A distinctive component consisting of two sugar alcohol phosphates, ribitol phosphate, is integral to the sugar chain and is essential for matriglycan elongation. FKTN, serving as a ribitol phosphate transferase, is crucial to the process. In individuals with FCMD, the inability to form ribitol phosphate structure prevents matriglycan modification, resulting in the onset of muscular dystrophy.
DGpathy is frequently associated with central nervous system abnormalities, including mental retardation and brain malformations (type II lissencephaly), and with cardiomyopathy. Upon injury, myofibers undergo regenerated through the activation of satellite cells, a type of stem cell. These satellite cells differentiate into muscle progenitor cells and myoblasts, ultimately fusing into myotubes. In myofiber-selective muscle creatine kinase (MCK)-FKTN conditional knockout (cKO) mice, myofibers exhibit susceptibility to mechanical stress, although the resulting muscle pathology is relatively mild. In contrast, muscle progenitor cell-selective Myf5-FKTN cKO mice exhibited severe muscular dystrophy. Satellite cells prepared from Myf5-FKTN cKO mice show decreased proliferative and differentiation potentials, resulting in decreased muscle regenerative capacity (1). Therefore, diminished muscle regenerative capacity may contribute to the severe muscle pathology observed in DGpathy. DG sugar chains are important for maintaining the glia limitans-basement membrane complex during fetal brain development. Disruption of the glia limitans-basement membrane complex, resulting from abnormal glycosylation, and neuronal overmigration from the affected areas are considered contributing factors to brain malformations (2). It has also been suggested that DG is involved in inhibitory synaptic function, axonal guidance, and the structure and function of glial end-feet, and that these abnormalities may be involved in central nervous system pathology. In cardiac muscles, the DG sugar chain is involved in maintaining the physical strength of myocyte membranes against mechanical overload and in the adaptive hypertrophic response to mechanical loading (3). Since each disease classified as DGpathy is a single-gene disorder such as FCMD, gene replacement therapy may be an effective treatment. Myofiber-specific FKTN gene transfer using an adeno-associated virus vector and the MCK promoter in Myf5-FKTN cKO mice showed therapeutic effects (1). This result suggests that, even if the muscle regeneration process is impaired, targeting the fragility of the myofiber cell membrane, which triggers the onset of the disease, can achieve therapeutic effects. The majority of FCMD patients have transposon insertion mutations in the 3' untranslated region of FKTN. Transposon insertion causes abnormal splicing; thus the normal FKTN protein is not produced. It has been reported that administration of antisense nucleotides, which can correct this abnormal splicing, recovers normal FKTN protein production in FCMD model mice and patient cells, which may lead to therapy (4). Ribitol phosphate transferases use cytidine diphosphate (CDP)-ribitol as a donor substrate. CDP-ribitol synthesis is facilitated by isoprenoid synthase domain-containing (ISPD), also known as CDP-L-ribitol pyrophosphorylase A (CRPPA). For DGpathy cases with ISPD mutations, there is impaired CDP-ribitol biosynthesis. Therefore, supplementation with CDP-ribitol is considered to be therapeutically effective. A recent report highlighted that administering the CDP-ribitol prodrug, capable of being delivered into muscle cells and converting back to active CDP-ribitol, improves muscle pathology in ISPD-deficient mice (5). As mentioned above, various therapeutic strategies for DGpathies have been proposed over the past decade based on causative gene functions and molecular pathomechanisms. The day is not far off when an effective treatment will be established for DGpathies, which has long been considered an intractable disease with no cure. Motoi Kanagawa
| |||||||||||||
| References | |
|---|---|
| (1) | Kanagawa M, Yu CC, Ito C, Fukada S, Hozoji-Inada M, Chiyo T, Kuga A, Matsuo M, Sato K, Yamaguchi M, Ito T, Ohtsuka Y, Katanosaka Y, Miyagoe-Suzuki Y, Naruse K, Kobayashi K, Okada T, Takeda S, Toda T: Impaired viability of muscle precursor cells in muscular dystrophy with glycosylation defects and amelioration of its severe phenotype by limited gene expression. Hum. Mol. Genet. 22, 3003-3015, 2013 |
| (2) | Sudo A, Kanagawa M, Kondo M, Ito C, Kobayashi K, Endo M, Minami Y, Aiba A, Toda T: Temporal requirement of dystroglycan glycosylation during brain development and rescue of severe cortical dysplasia via gene delivery in the fetal stage. Hum. Mol. Genet. 27, 1174-1185, 2018 |
| (3) | Ujihara Y, Kanagawa M, Mohri S, Takatsu S, Kobayashi K, Toda T, Naruse K, Katanosaka Y: Elimination of fukutin reveals cellular and molecular pathomechanisms in muscular dystrophy-associated heart failure. Nat. Commun. 10, 5754, 2019 |
| (4) | Taniguchi-Ikeda M, Kobayashi K, Kanagawa M, Yu CC, Mori K, Oda T, Kuga A, Kurahashi H, Akman HO, DiMauro S, Kaji R, Yokota T, Takeda S, Toda T: Pathogenic exon-trapping by SVA retrotransposon and rescue in Fukuyama muscular dystrophy. Nature 478, 127-131, 2011 |
| (5) | Tokuoka H, Imae R, Nakashima H, Manya H, Masuda C, Hoshino S, Kobayashi K, Lefeber DJ, Matsumoto R, Okada T, Endo T, Kanagawa M, Toda T: CDP-ribitol prodrug treatment ameliorates ISPD-deficient muscular dystrophy mouse model. Nat. Commun. 13, 1847, 2022 |
Mar. 15, 2024