|
Mammalian Fertilization and Sugars
|
|
|
 |
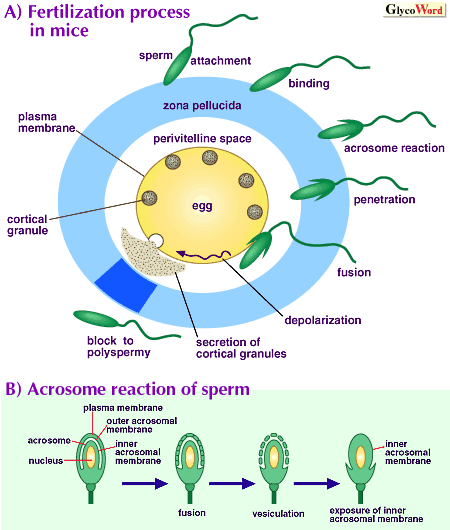
Various interactions between sperm and eggs are involved in mammalian fertilization (figure). First, capacitated sperm loosely adhere and then tightly bind to the zona pellucida surrounding eggs. This binding is mediated by specific interaction between ZP glycoproteins and egg-binding proteins on the sperm surface, and induces the acrosome reaction which involves fusion and vesiculation of outer acrosomal membrane and sperm plasma membrane at many sites, release of the vesicles, and exposure of the inner acrosomal membrane. Then, the acrosome-reacted sperm penetrate the ZP, reach the perivitelline space, and fuse with the egg. To block polyspermy, transient depolarization of the egg plasma membrane and release of cortical granules including various enzymes to the perivitelline space are induced immediately after sperm-egg fusion, resulting in physical and chemical alterations of the ZP and inactivation of its sperm-binding ability. |
|
|
 |
|
|
Thus, ZP plays important roles in sperm-egg binding, induction of sperm acrosome reaction, and blocking of polyspermy. However, its composition is rather simple. ZP is composed of three glycoproteins called ZP1, ZP2 and ZP3 in mice, and of three glycoproteins called 90K, 55K alpha and 55K beta in pigs. Human, monkey, hamster and rat ZP are also composed of only 2 to 4 different glycoproteins. ZP3 is responsible for binding to acrosome-intact sperm and ZP2 for binding to acrosome-reacted sperm in mice, and a complex of 55K alpha and 55K beta seems to be responsible for more tight sperm-egg binding in pigs. Structures of N and O-linked oligosaccharides of porcine ZP and N-linked oligosaccharides of mouse and bovine ZP have so far been elucidated.
Fertilization is basically species-specific. For example, human sperm do not bind to mouse and hamster eggs. This is considered due to different specificity in binding of sperm to the oligosaccharides on ZP glycoproteins. Recently, it has been shown that mouse sperm, but not human sperm, bind to eggs of genetically altered mice expressing human ZP3 but lacking mouse ZP3 in vitro and fertility is observed in vivo, and the significant role of oligosaccharides with defined structures in species-specific fertilization is noted again. Extensive studies on the sugar recognition mechanism involved in fertilization have been performed in mice, and two hypotheses have been proposed. One is a proposal by Wassarman that O-linked oligosaccharides of ZP3 with alpha-galactosyl residues at their non-reducing termini mediate sperm-binding to egg, and sperm protein called sp56 has been suggested as a complementary sugar-binding protein. However, there are conflicting reports that alpha-galactosyl transferase-deficient mice are fertile, and that sp56 is not exposed on the sperm cell surface. The other is a proposal by Shur that sperm-egg binding is mediated by interaction between sperm surface beta-galactosyltransferase and its putative O-linked sugar chains of ZP3 with non-reducing terminal GlcNAc residues. Recently, beta-galactosyltransferase-I knockout mice have been produced. Their sperm bind less ZP3 (30% to 50% of the wild-type sperm) and are not able to undergo ZP3-induced acrosome reaction in vitro; however, knockout males are still fertile in vivo. Contrary to the above two proposals, there is a report showing that the system recognizing beta-galactosyl residues of oligosaccharides on ZP is working in the sperm-egg interaction. Thus, the carbohydrate recognition mechanism in fertilization is not clearly elucidated even in the well-studied mouse system.
There is no doubt about that sugar chains play an important role in fertilization, but there are many problems to be solved: identification and characterization of sugar chains working as sperm receptors and sugar recognizing complementary proteins on the sperm membranes, and so on. Elucidation of these problems is important to the understanding mechanisms by which species-specific fertilization and block to polyspermy are regulated. |
|
|
|
Seiichi Takasaki (Department of Biochemistry, Institute of Medical Science, University of Tokyo) |
|
|
|
|
|
| References |
(1) |
Wassarman, PM : Profile of a mammalian sperm receptor. Development.108, 1-17, 1990 |
|
(2) |
Shur, BD : Cell surface galactosyltransferase: current issues. Glycoconjugate J. 15, 537-548, 1998 |
|
(3) |
Mori, E, Mori, T, Takasaki, S : Mouse sperm bind to beta-galactose residues on egg zona pellucida and asialofetuin-coupled beads. Biochem. Biophys.Res. Commun. 238, 95-99, 1997 |
|
|
|
|
|
| Dec.15, 1998 |
|
|
|
|
|
|
|



