|
Sialylmotif
|
|
|
 |
Sialyltransferases (STs) are a family of glycosyltransferases that catalyze the transfer of sialic acid residues from CMP-sialic acids as donor substrates to the carbohydrate groups of glycoproteins and glycolipids as acceptor substrates according to the following reaction.
CMP-sialic acid + HO-R --> CMP + sialyl-O-R
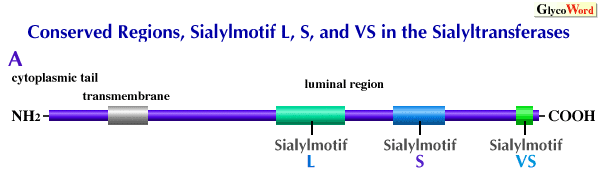
All members of the sialyltransferase genes cloned to date have three conserved regions in the luminal region, namely sialylmotif L(long), sialylmotif S (short), and sialylmotif VS(very short) (Figure, Ref. 1, 2). |
|
|
 |
 |
 |
 |
 |
 |
 |
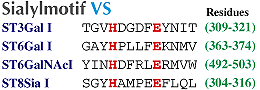 |
 |
|
| Figure Legend |
A, Schematic structure of sialyltransferases showing the relalive positions of sialylmotif L,S, and VS.
B, Amino acid sequence of sialylmotif L, S, and VS in four sialyltransferases. Consensus amino acid residues in the all sialyoltransferases are shown by bold letters. |
|
|
|
|
Based on the observation that when ST protein was mutated within sialylmotif L by site-directed mutagenesis, its Km value for the CMP-sialic acid was increased as compared with that of wild type ST, sialylmotif L is believed to participate in the binding of the donor substrate, CMP-NeuAc (3). A recent report showing that mutation of the sialylmotif S caused change in Km values for both the donor and acceptor substrates suggests that sialylmotif S is involved in the binding of both substrates (4). Even though an adidtional conserved motif is also observed at COOH-terminal region of ST genes and designated as sialylmotif VS, the functional role of this motif remains unknown (2). Structural analysis of sialyltransferases by crystallography has been anticipated to better understand how these domains are involved in catalytic reactions.
Taking advantage of the presence of these conserved regions in all sialyltransferase genes, additional members of sialyltransferase genes have been successfully cloned by PCR technique (5). |
|
|
|
Shinobu Kitazume-Kawaguchi (RIKEN institute) |
|
|
|
| References |
(1) |
Tsuji, S, Datta, A, Pauson, J C : Systematic nomenclature for sialyltransferases. Glycobiology, 6, v-vii. 1996 |
|
(2) |
Geremia, RA, Harduin-Lepers, A, Delannoy, P : Identification of two novel conserved amino acid residues in eukaryotic sialyltransferases: implications for their mechanism of action. Glycobiology, 7, v-vii. 1997 |
|
(3) |
Datta, A, Paulson, J C : The sialyltransferase"sialylmotif" participates in binding the donor substrate CMP-NeuAc. J.Biol. Chem. 270, 1497-1500, 1995 |
|
(4) |
Datta, A K, Sinha, A, Paulson, J C : Mutation of the sialyltransferase S-sialylmotif alters the kinetics of the donor and acceptor substrates. J. Biol. Chem. 273, 9608-9618, 1998 |
|
(5) |
Tsuji, S : Molecular cloning and functional analysis of sialyltransferases. J. Biochem. 120, 1-13.1996 |
|
|
|
|
|
|
| Sep.15, 1998 |
|
|
|
|
|
|
|



