| A beta-1,4-Galactosyltransferase Family | |
|
 |
Most biologically active carbohydrate antigens are expressed on the Gal(beta)1 -->3/4GlcNAc groups which are often found in the outer chain moieties of glycoconjugates. The beta-galactosyltransferase (beta-GalT) synthesizes the disaccharide groups by transferring Gal from UDP-Gal to GlcNAc residues on the sugar chains. The cDNAs encoding the previously unknown beta-1,4-GalTs and beta-1,3-GalTs have recently been cloned one after another, and it turns out that the members of beta-1,4-GalTs and beta-1,3-GalTs form individual families. Although they share the sugar-donor and sugar-acceptor, there is no sequence similarity between these families.
In 1986, beta-1,4-GalT cDNA was isolated from bovine kidney and mammary gland cDNA libraries. Since no homologous gene was cloned, many scientists believed that beta-1,4-linked Gal residues found in glycoconjugates could be formed by beta-1,4-GalT (beta-1,4-GalT I). Targeted inactivation of mouse beta-1,4-GalT I gene, however, revealed that the tissue and serum glycoproteins contain beta-1,4-linked Gal residues in the N-linked oligosaccharides and that the residual beta-1,4-GalT activity towards glycoprotein acceptors is present in the tissue homogenates. These results indicate that there are another yet unknown protein(s) with beta-1,4-GalT activity in animal cells.
Recently novel human beta-1,4-GalT genes have been cloned by using a highly conserved amino acid sequence found in the putative catalytic domains of glycosyltransferases including beta-1,4-GalT I as a primer (1) and by the sequence information from the EST and UniGene data banks (2,3). Since they show identities of 55, 44, 41, 37, and 33% to beta-1,4-GalT I at an amino acid level, they are referred to as beta-1,4-GalT II, III, IV, V and VI, respectively, based on the homology distance in this section. Note that beta-1,4-GalT V described in this section corresponds to beta-1,4-GalT IV originally described in reference 1. To date, among a human beta-1,4-GalT family mouse and chicken beta-1,4-GalT I genes and chicken beta-1,4-GalT II gene have been cloned. Quite recently lactosylceramide synthetase has been purified from rat brain and its cDNA has been cloned based on a partial amino acid sequence (4). The deduced amino acid sequence revealed that it is almost identical to human beta-1,4-GalT VI.
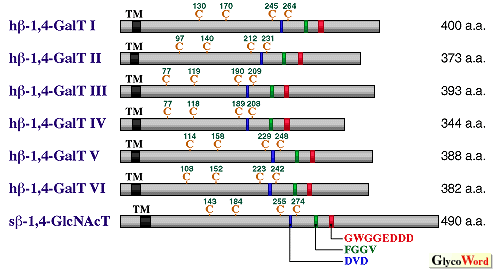
Multiple sequence alignment of beta-1,4-GalT family members is shown in Fig.1. High sequence identities as well as similarities are observed in the putative catalytic domains located at the C-terminal regions of individual transferases. The regions including the DVD, FGGV and GWGEDDD sequences have also been found in beta-1,4-N-acetylglucosaminyltransferase (beta-1,4-GlcNAcT) whose cDNA was isolated from snail, indicating that these peptide motifs could be involved in binding to sugar-acceptor and/or a part of sugar-donor (UDP), which are utilized by the transferases forming a beta-1,4-linkage. The relative positions of four cysteine residues in each glycosyltransferase shown in Fig. 1 are also well preserved among the members of this family. In spite of sharing these homologous domains, the substrate specificities of beta-1,4-GalTs appear to be different from each other. The beta-1,4-GalT I, II and V are considered to be involved in the galactosylation of glycoprotein sugar chains, while beta-1,4-GalT III and VI could be involved in glycolipid synthesis, lacto-N-neotetraosylceramide and lactosylceramide, respectively. In fact, the expression levels of beta-1,4-GalTs in tissues and the localization of each beta-1,4-GalT in cells are more important for their functions than their in vitro acceptor specificities.
| |
|
 |
|
Fig. 1
Schematic representation of human beta-1,4-GalT family members and snail beta-1,4-GlcNAcT. TM indicates the putative transmembrane domain. Cystein (C) residues are indicated in orange. The h and s indicate human and snail.
| |
|
The expression levels of individual beta-1,4-GalT transcripts among human tissues (heart, brain, placenta, lung, liver, muscle, kidney, and pancreas) were examined by Northern blot analysis. The beta-1,4-GalT I, III, IV and V mRNAs were expressed in most tissues. In contrast, beta-1,4-GalT II mRNA was expressed in heart, muscle and pancreas, and beta-1,4-GalT VI mRNA was expressed only in brain. The beta-1,4-GalT I is involved in lactose synthesis by transferring Gal to Glc in the presence of alpha-lactalbumin. In mouse mammary gland, the transcript of beta-1,4-GalT I was highly expressed but not of other beta-1,4-GalTs.
Study of the detailed acceptor specificities of the individual beta-1,4-GalTs just started and the results to be obtained will provide us how important these newly discovered beta-1,4-GalTs are in our living cells.
| |
|
| Takeshi Sato (Department of Biosignal Research, Tokyo Metropolitan Institute of Gerontology) | |
|
|
| References | (1) | Sato, T, Furukawa, K, Bakker, H, Van den Eijnden, D H, Van Die, I : Molecular cloning of a human cDNA encoding a novel beta-1,4- galactosyltransferase with 37% identity to mammalian UDP-Gal:GlcNAc beta-1,4-galactosyltransferase. Proc. Natl. Acad. Sci. U.S.A. 95, 472-477,1998. |
| (2) | Almeida, R, Amado, M, David, L, Levery, SB, Holmes, E, Merkx, G, Van Kessel, AG, Rygaard, E, Hassan, H, Bennett, E, Clausen, H: A family of human beta4-galactosyltransferases : Cloning and expression of two novel UDP- galactose:beta-N-acetylglucosamine beta1,4-galactosyltransferases, beta4Gal-T2 and beta4Gal-T3. J. Biol. Chem. 272, 31979-31991, 1997. |
| (3) | Lo, N- W, Shaper, J H, Pevsner, J, Shaper, N L : The expanding beta4-galactosyltransferase gene family: message from the databanks. Glycobiology, 8, 517-526, 1998. |
| (4) | Nomura, T, Takizawa, M, Aoki, J, Arai, H, Inoue, K, Wakisaka, E, Yoshizuka, N, Imokawa, G, Dohmae, N, Takio, K, Hattori, M, Matsuo, N : Purification, cDNA cloning, and expression of UDP-Gal: glucosylceramide beta-1,4-galactosyltransferase from rat brain. J. Biol. Chem. 273,13570-13577, 1998. |
| | |
| |
|
| Sep.15, 1998 |
|
| |
|
|
|
|



