

 |
 |
N-linked Oligosaccharide as a Signal for Proteolysis |
 |
||||||||||||||||
 |
Carbohydrates have been recognized as the face of cells and implicated in many biological functions particularly on the outside of cells. However, it has been elucidated that carbohydrates play important roles not only outside, but also inside cells. Here, the quality control of glycoproteins is summarized, focusing on the role of N-linked oligosaccharides as the signal of glycoprotein degradation. Secretary proteins including membrane proteins, secretion proteins and lysosomal enzymes, all of which are synthesized in the ribosome, are translocated into the endoplasmic reticulum (ER). These nascent proteins are then subjected to extensive modification as mature glycoproteins and move through the ER via the Golgi complex to their final destinations inside and outside cells. In the ER, the N-linked oligosaccharides are homogeneous and relatively simple, containing high-mannose oligosaccharides. There are no species differences from yeast to mammalians. It has been well documented that Calnexin and Calreticulin, both of which are unique chaperons, recognize Glc1Man9GlcNAc2. N-linked oligosaccharides of folded proteins properly with ER chaperons are processed and trimmed, and these proteins having high-mannose oligosaccharides then move to the Golgi complex, where N-linked oligosaccharides are further processed and trimmed, and finally converted to complex and hybrid types, both of which have sialic acid residues at the non-reducing termini with microheterogeneiety. On the other hand, misfolded proteins and unassembled polymer proteins are translocated from ER into cytosol via Sec61, where these proteins are degraded by the ubiquitin-proteasome system. This mechanism is termed ER-associated degradation (ERAD). This area of research is in full bloom showing that N-linked oligosaccharides are involved in the ERAD. In an attempt to obtain proteins that bind to carbohydrates in the central nervous system, a novel ubiquitin ligase, Fbx2, was detected in the cytosol fraction of mouse brain extracts (1). Extensive studies revealed that (i) the Fbx2 binds specifically to proteins with high-mannose oligosaccharides, (ii) pre-integrin The failure of ERAD is considered to be a major cause of neuronal degenerative disorders such as Altzheimer's and Parkinson's diseases. Several lines of evidence show that Fbx2 is expressed in neuron, predominantly in adult brain, and may play a role in protein quality control (1). Thus, it is expected that further studies on Fbx2 and its homologues may develop and advance a new therapy for neuronal degenerative diseases. |
||||||||||||||||
 |
|||||||||||||||||
|
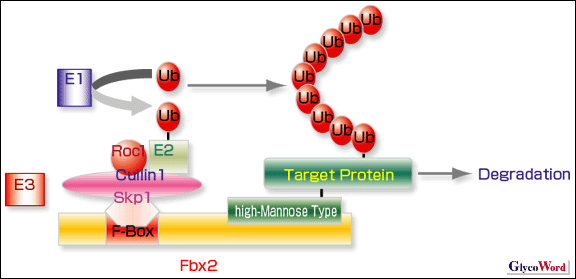
Fig.1. The SCF ubiquitin ligase complex.
The ubiquitin system is composed of three enzymes, the activating enzyme (E1), the conjugating enzyme (E2), and the ligase (E3), and a ubiquitin (Ub) is covalently attached to a target protein. E3 forms the Skp1-Cullin1-F-box Protein-Roc1 ubiquitin ligase complex (SCF complex). A F-box protein, Fbx2, recognizes high-mannose oligosaccharides and poly-ubiquitinates a target protein, which is finally degraded by the 26S proteasome. |
|||||||||||||||||
| Tadashi Tai (The Tokyo Metropolitan Institute of Medical Science, Tumor Immunology) | |||||||||||||||||
|
|||||||||||||||||
| Mar.31, 2004 | |||||||||||||||||
|
|
|||||||||||||||||
|
|||||||||||||||||