|
Frontal Affinity Chromatography for Quantitative Analysis of Sugar-Protein Interaction
|
|
|
 |
Frontal affinity chromatography (FAC) is a quantitative method developed by Kasai et al., which can accurately determine affinity constants between biomolecules such as enzymes-substrate analogues, lectin-oligosaccharides. Other methods for this purpose include classic equilibrium dialysis, and more recently, biosensor techniques represented by BIAcore. However, most of these methods have difficulty as regarding accuracy, simplicity or economy. In this context, FAC is superior in its clarity of principle and the simplicity of its system (see Fig. 1). The latter greatly contributes to cost effectiveness. The basic equation (1) of FAC is essentially equivalent to that described for enzyme kinetics, i.e., the Michaelis-Menten equation: two variables in the latter equation, i.e., substrate concentration "s" and reaction velocity "v", correspond to [A]0 (initial concentration of A) and V-V0 (retarded volume of elution), respectively, where V0 is the elution volume of an appropriate substance that has no affinity to B. By analyzing changes in V-V0 vs. [A]0 by means of Lineweber-Burk type double reciprocal plot (eq. 2), ligand content Bt (corresponding to maximum reaction velocity Vmax in enzyme kinetics) and dissociation constant Kd (in M, corresponding to Michaelis constant KM) are obtained. Further, FAC is more advantageous than other methods for the analysis of relatively weak interactions. Thus, the method is most suitable for analysis of sugar-protein interaction, which is in general weaker then that for antigen-antibody. |
|
 |
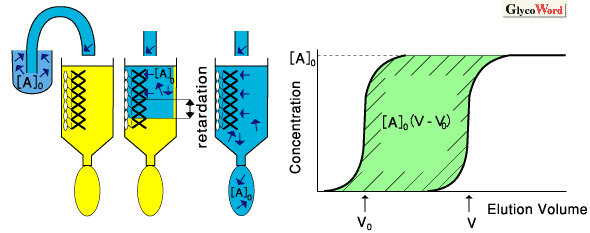 |
Fig.1
Principle of FAC: An initial concentration ([A]0) of "A" (e.g., labeled sugar) is continuously applied to a column, where affinity ligand "B" (e.g., lectin) is immobilized. If "A" has no affinity to "B," the elution front of "A" is observed at V0, whereas if "A" has significant affinity to "B," elution front of "A" is significantly retarded to the point V. When [A]0 is small enough ([A]0<< Kd), the retarded volume V-V0 becomes proportional to the affinity constant (inverse of the dissociation constant) by eq. (3). |
|
|
|
|
 |
|
|
|
Despite the above merits, previous FAC required a relatively long time and a large amount of sample for analysis because conventional open-type columns were used. Recently, these were greatly improved by utilizing HPLC in a few laboratories. Hirabayashi et al. reinforced the FAC system by the use of a miniature column (bed volume, 0.126 ml) packed with lectin or glycoprotein-immobilized resin, to which fluorescent-labeled oligosaccharides or lectin proteins, respectively, were applied at a constant flow rate and concentration via a relatively large sample loop (Fig. 2). When pyridylaminated oligosaccharides (available from Takara and Seikagaku Kogyo) are used, 2 ml of 10 nM solution is enough for detection, and it takes only 10 min for each analysis. Examples of FAC analysis using a Caenorhadbitis elegans (C. elagans) galectin (LEC-6) column with several pyridylaminated sugars are shown in Fig. 3. In a previously study, elution front of each oligosaccharide was determined graphically. However, upon total miniaturization by incorporating various HPLC equipment, it because necessary to develop a more accurate procedure to determine V value. For this purpose, Arata et al. reported a data-processing procedure utilizing the table-calculating software, Microsoft Excel.
|
|
|
|
| fig.2 A system for improved FAC |
|
|
|
|
Fig. 3
Examples of FAC analysis: C. elegans galectin LEC-6 is immobilized at a concentration of 7.44 mg/ml gel, and to this column 6 pyridylaminated oligosaccharides derived from glycolipids (10 nM) are applied through a 2-ml sample loop at a flow rate of 0.25 ml/min. Rhamnose is used as a negative control to obtain V0. Kd for each oligosaccharide is calculated according to eq. (1) by using V-V0 and Bt values determined by concentration analysis with respect to p-aminophenyl-b-lactoside. |
|
|
|
FAC can be carried out by using either lectin columns to which labeled glycans are applied, or inversely by using glycan columns to which lectin proteins are applied. However, the former is more generally used and successful, because a panel of oligosaccharides can be analyzed for comparison. When lectin solutions are applied to glycan columns, as low as 1 mg/ml of lectin protein is detectable if it contains fluorescent tryptophan. In this case, only a few micrograms of protein is required for each analysis. On the other hand, upon immobilization, some points must be considered, e.g., lectin stability and oligomerization. In particular, the formation of multivalent linkages between lectin and matrix gel (e.g., Hi-Trap NHS-activated, Pharmacia) should be avoided, since they may significantly lower the column capacity in terms of Bt. In such a case, the inclusion of an appropriate amine such as Tris may improve the Bt value. In this context, information an the Lys distribution of immobilized lectin will also be necesary. The proportion of Bt relative to immobilized ligand content is defined as "availability," which usually ranges between 20 and 60%.
For the determination of Kd and Bt, either Lineweber-Burk (i.e., 1/[A]0 vs. 1/[A]0(V-V0)) or Woolfe-Hofstee type plots (i.e., V-V0 vs. [A]0(V-V0) are performed by varying [A]0 (in general the latter gives more reliable results besides poorer linearity of the regression line). From a practical viewpoint, however, rather few labeled saccharides, e.g. p-aminophenyl-ß-lactoside are available for this purpose. Alternatively, the concentration of non-labeled saccharide may be varied with a constant concentration of the labeled one, if both of them have the same affinity to the immobilized lectin. If [A]0 is negligibly small compared with Kd (Kd >> [A]0 ), V should reach a maximum value, independent of [A]0, and thus, eq. (3) is obtained. The equation means that the affinity between the immobilized lectin and a saccharide is simply proportional to the value of V-V0.
|
|
|
|
|
|
Jun Hirabayashi (Teikyo University, Faculty of Pharmaceutical Sciences) |
|
|
|
|
|
| References |
(1) |
Kasai K, Oda Y, Nishikawa S, Ishii S : Theory for its application to studies on specific interaction of biomolecules. J. Chromatogr. 376, 33-47, 1986
|
|
(2) |
Hirabayashi J, Arata Y, Kasai, Y : Reinforcement of frontal affinity chromatography for effective analysis of lectin-oligosaccharide interactions. J. Chromatogr. A, 890, 261-271, 2000
|
|
(3) |
Arata Y, Hirabayashi J, Kasai K : Sugar binding properties of the two lectin domains of the tandem repeat-type galectin LEC-1 (N32) of Caenorhabditis elegans. J. Biol. Chem. 276, 3068-3077, 2001
|
|
(4) |
Schriemer DC, Bundle DR, Li L, Hindsgaul O : Micro-scale frontal affinity chromatography with mass spectrometric detection: a new method for the screening of compound libraries. Angew. Chem. Int. Ed. 37 (1998), 3383-3387, 1998
|
|
|
|
|
|
| Jun. 15, 2001 |
|
|
|
|
|
|
|



