|
Basement Membrane Proteoglycans
|
|
|
 |
Basement membranes are defined electron microscopically as characteristic sheet-like structures of extracellular matrix which are formed in the interface between parenchymal cells and their surrounding connective tissues. They are ubiquitously distributed in the body, while those in glomerular capillary loops or synaptic membranes are highly differentiated with their unique structures. The basement membrane serves as not only a tissue boundary on which cells attach but also a filter with selective permeability or a highly specialized substrate for cellular differentiation and gene expression. The negative charge of the glomerular basement membrane (GBM) has been attributed to the presence of heparan sulfate proteoglycan (HSPG) called perlecan, which is widely distributed in various kinds of basement membranes, in addition to GBM. Other classical constituents of the basement membrane are type IV collagen, laminin, and entactin.
There are several HSPG molecules in the basement membrane other than perlecan which have been best characterized, namely, agrin and smaller HSPGs. In addition to HSPGs, bamacan, a chondroitin sulfate proteoglycan, is known to be another anionic component of the basement membrane.
1. Perlecan
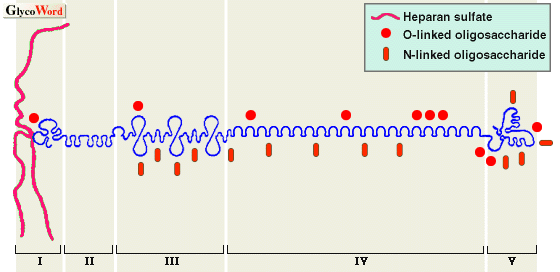
Perlecan is a major HSPG molecule of the basement membrane. However, it is also expressed in embryonic interstitial tissues and stromata in pathologic conditions. The core protein of perlecan, 467 KDa (human), is composed of five domains. The amino-terminal domain I contains three heparan sulfate (HS) chains; domain II has four LDL receptor-like repeats; domain III contains repeats resembling those in the short arm of laminin; domain IV has Ig-like repeats similar to those in NCAM, and domain V contains repeats similar to those in laminin A chain and EGF motifs. Such a domain structure suggests that the core protein itself carries multifunctional properties, although there is little actual evidence for them. Its HS chains are heterogeneous in size; however, each HS chain averaged 380 KDa in molecular mass and 87 nm in length when examined by electron microscopy. A hybrid variant of perlecan with dermatan sulfate chains has been reported. Like other HSPG molecules, various functions of HS chains including binding with several growth factors are being revealed. In addition to HS chains, perlecan bears a total 20 KDa of N- and O-linked oligosaccharide chains, which are suggested to function in the secretion of perlecan.
2. Agrin
Agrin was found as a protein which induces clustering of acetylcholine receptors and acetylcholine esterase at the postsynaptic membrane of neuromuscular junctions. Recently, it has been shown to be a HSPG and distributed in basement membranes other than synapses. In the GBM, agrin is much more abundant than perlecan. The core protein of agrin, 250 KDa (chicken), is composed of the amino-terminal globular head containing laminin binding sites, a second rod-like domain resembling follistatin, and a third globular domain containing laminin G. It is predicted that it has four each of HS chains and N-linked oligosaccharide chains. The HS chains have been shown to bind to NCAM in nervous tissues. Agrin isoforms localized in synaptic sites are products of special alternative splicing.
3. Bamacan
Bamacan, a chondroitin sulfate (CS) proteoglycan of the basement membrane, is localized in various kinds of basement membranes except for the glomerular capillary loop. The core protein of bamacan, 138 KDa (mouse), consists of five domains: the amino-terminal globular head, the second rod attached with one each of CS chain and a N-linked oligosaccharide chain, the third globular, the fourth rod containing two N-linked oligosaccharide chains, and the fifth globular tail with two CS chains. The function of bamacan has not been well elucidated yet. |
|
|
| Fig. 1. Schematic molecular structure of perlecan |
 |
|
|
|
|
Takashi Saku (Niigata University School of Dentistry) |
|
|
|
| References |
(1) |
Noonan, DM, Hassel, JR: Proteoglycans of basement membranes. In: Rohrbach, DH, Timpl, R (ed): Molecular and cellular aspects of basement membranes. Academic Press, Inc, New York, pp. 189-210, 1993 |
|
(2) |
Tsen, G, Halfter, W, Kroeger, S, Cole, G: Agrin is a heparan sulfate proteoglycan. J. Biol. Chem. 270, 3392-3399, 1995, |
|
(3) |
Wu, R, Couchman, JR: cDNA cloning of the basement membrane chondroitin sulfate proteoglycan core protein, bamacan: a five domain structure including coiled-coil motifs. J. Cell Biol. 136, 433-444, 1997 |
|
|
|
|
|
|
| Sep.15, 1998 |
|
|
|
|
|
|
|



