
ProfessorUlf Lindahl
Dr. Ulf Lindahl was born 1940 in Solna, Sweden. He received his MD/PhD from Uppsala University in 1966. His thesis was 'Structure of the heparin-protein linkage region'. He studied at LaRabida-Univ. of Chicago as a Research Student Assistant 1962-64. He returned to Uppsala University in 1965 and was appointed as the Ass. Prof. at dept. of Medical and Physiological Chemistry in 1967. He became a Career investigator at the Medical Research Council in 1971. Dr. Lindahl moved to Royal Veterinary College; Swedish University of Agricultural Sciences as the Professor in Medical and Physiological Chemistry in 1973, and became Chairman of the department for 8 years there. He returned to Uppsala University in 1991 as the Professor in Medical and Physiological Chemistry. He became the chairman of Dept. of Medical Biochemistry and Microbiology at Uppsala University in 1998. His numerous honors include: the Chairman of Study Section for Chemistry in the Swedish Medical Research Council and the member of the Committee for Molecular Biology, 1986-92; the Secretary to the National Committee for Biochemistry, 1988-90. Achievements include: The Anniversary Prize of the Gesellschaft fur Biologische Chemie (FEBS, 1980); the Hilda and Alfred Erikssons Prize from the Royal Swedish Academy of Sciences (1984); the International Karl Meyer Award of the Society for Complex Carbohydrates (1991); the Anders Jahres Store Nordiske Medisinske Pris (1993); the Owren Lecturer (Oslo,1989); Honorary Memberships of 5 learned societies; editorship of 8 journals; Author of about 200 scientific papers, mainly concerned with the structure, biosynthesis and function of heparin and related polysaccharides.
In recent years, the sugar chain of heparan sulfate proteoglycans has attracted glycoscientists because of its diverse functions. Heparan sulfate proteoglycans on the cell surface bind and modulate biological activities of various growth factors, enzymes and protease inhibitors. While, in the matrix, they bind cells, preserve physiological proteins, and regulate selective transport through the basement membranes.
Heparan sulfate is a high molecular weight polysaccharide being diversely modified by sulfation. It might be one of the most interesting subjects in modern glycoscience to elucidate their structures related to such various functions and the systems of its biosynthesis.
Professor Ulf Lindahl has been one of the most experienced researchers and a prominent leader of heparin studies since the beginning of this research field. Among many of his achievements, the determination of the domain structures of the heparin having anticoagulant activities has made an epoch in the researches on the functional domains in heparin/heparan sulfate chains. The present day studies on heparan sulfate biosynthesis are critically influenced by his comprehensive studies on heparin biosynthesis.
This interview was intended to learn about the motive and history of Dr. Lindahl's heparin studies, and his thoughts on the critical subjects in heparan sulfate studies now and in the future.
QDr. Lindahl, you have been devoted to the heparin studies for a long time and made a large contribution to this field. What was your leading motive for studying heparin?
I would say it was just a lucky chance. I went to medical school in Uppsala. Lennart Roden, who gave a class in organic chemistry, happened to be the supervisor of my seminar group. I ran a few Sephadex columns under his guidance, and then he took off to University of Chicago, where he did most of his work on polysaccharide-protein linkage regions. After some time he wrote and asked if I wanted to join him as a graduate student. I did, together with my wife, Birgitta, and we started to work on the origin of covalently bound amino acids in polysaccharide preparations. So, together with Lennart, I found the heparin-protein linkage region. It seemed natural to look also at the more peripheral regions of the heparin chain. Then, with time, as the relation between heparin and heparan sulfate (HS) became more obvious, the whole field expanded enormously. For me, it turned out to be enough for a life-time of research.
QIsolation of the anticoagulantly active heparin and characterization of its domain structure had striking effects on the subsequent heparin and heparan sulfate studies. How and why did you initiate this investigation?
It was common knowledge since way back that heparin was able to bind to various proteins. The first heparin-Sepharose column for protein fractionation purposes was made at our department in the early 70's (by Per-Henrik Iverius). Previously, Ulrich Abildgaard had identified "heparin cofactor" as antithrombin, and Robert Rosenberg subsequently showed that antithrombin, by binding to heparin, is converted into a much more efficient inhibitor of the coagulation proteases. This was a key discovery. It seemed natural to ask whether all heparin molecules had this ability, and so we tried affinity chromatography on immobilized antithrombin. Similar experiments were conducted, almost simultaneously, by Rosenberg in Boston, and by Lars-Olov Andersson and his coworkers at Kabi Pharmaceutical in Stockholm. All three groups obtained the same result; only a minor fraction of the heparin molecules bound with high affinity to antithrombin, and this fraction was responsible for almost all of the anticoagulant activity of the starting material. It took us another four years to define the structural difference between the high-affinity and low-affinity forms of heparin, maybe not so strange considering that this difference was due to a single sulfate group. The finding of this "unique" glucosamine 3-O-sulfate group in the antithrombin-binding pentasaccharide sequence has had interesting spin-off effects. In particular, one could mention the remarkable development of methodology for chemical synthesis of glycosaminoglycan structures carried out by Pierre Sinay and Maurice Petitou.
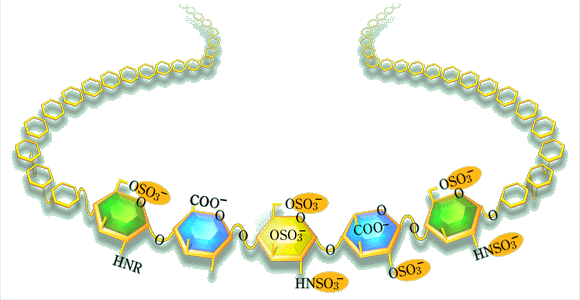
QHeparin has established an indisputable position in the medicine. However, its actual role in the living body seems to be still unclear. What is your opinion about it? Why does it exist only in mast cells?
A good question: what is heparin doing in the mast cell? As far as we know- which is not much - this function has nothing to do with disturbed blood coagulation. Heparin is discharged from the mast cell only in special emergency situations, such as anaphylactic shock or other inflammatory reactions. One possibility would be that the released heparin modulates an extravascular coagulation system that is switched on in inflammatory situations, and may result in extensive fibrin deposition in tissues. It is now well established that monocytes/macrophages may provide the required prothrombinase activity, when appropriately stimulated. Maybe we should look in quite different directions. I am thinking of the old finding of heparin-like polysaccharides in mast-cell-like cells in certain species of marine mussels. These creatures have no blood in the conventional sense to anticoagulate, yet the polysaccharide in the "mast cells" turns out to contain the specific antithrombin-binding pentasaccharide sequence. It also has very high anticoagulant activity. It seems reasonable to assume that the clams contain a protease inhibitor related to antithrombin (belonging to the serpin family), but for a functional reason quite unrelated to blood coagulation. Maybe a defense mechanism against parasites? Could the mammalian mast cells retain some similar function? In any case, it seems that the pentasaccharide/antithrombin interaction mechanism has been adopted to the regulation of blood coagulation in mammals, but through endothelial HS rather than heparin.
QFollowing the heparin studies, you proposed the fine structure model of the heparan sulfate domain interacting to b-FGF. Heparan sulfates are said to interact with diverse proteins and thereby to modulate their activities. How about the variability and the strictness of the domain structure of HS chains related to the interactions with these proteins?
This is a highly intriguing question, and one of the real "hot" issues in the field right now. Recent results obtained by us and others suggest that different cells and tissues express HSs with highly specific structural domains, that are presumably tailored to bind selected proteins. Of course, evidence in this regard is still indirect - we cannot isolate HS from a single cell type in a tissue, and polysaccharides produced by cultured cells may not be representative. Still, compositional analysis of HS preparations from different tissues indicate subtle but highly reproducible differences, consistent from one individual to another, that can only be interpreted in terms of tightly controlled biosynthetic modification of HS chains. Diverse expression of saccharide domains is also revealed through immunohistochemical examination of tissues, using monoclonal antibodies that recognize different (although still poorly characterized) HS epitopes. I am convinced that this variability in carbohydrate structure reflects the need for HS-based ligands in selective interactions with different proteins. I also believe that this insight has been delayed by the fact that most of these proteins bind heparin as well, in seemingly nonspecific fashion. The heavily sulfated heparin chain will encompass all the possibilities for protein binding that are differentially expressed by a multitude of HS species.
QThe diverse functions of HS tell us the importance of keeping these structures in living animals. How do you think about the biosynthetic system for synthesizing such specific HS and cells memorizing these molecular design?
How is HS, or heparin for that matter, synthesized? In my mind heparin, in the context of biosynthesis, should be regarded as just another HS, with unusually extended N-sulfated domains. This question is more of a challenge than ever, especially in view of our current understanding of HS structure, and the obvious need for regulation of its biosynthesis. I have been interested in this matter for decades and have, together with my coworkers, produced at least two partly contradictory models of the biosynthetic process. These models may seem a bit naive, but then I believe models can help develop your thinking around complex matters.
What has remained fairly well established during all this time is the importance of substrate specificity of the biosynthetic enzymes. The various polymer-modification reactions obviously take place in a given order, so that the product of one reaction will provide the substrate for the following one. The complex structures of HS arise through the incompleteness of these reactions; in each reaction step, say, of a particular sulfotransferase, some potential substrate residues appear to escape modification. The mechanism of substrate selection is still obscure. It will be most interesting to see how much of the mystery will in the end be explained by the occurrence of genetically distinct enzyme isoforms with distinct substrate specificity. Is there a "template" or "blueprint" to control the process? Is the HS chain made all at once, like heparin, which seems to be generated in less than a minute? I am convinced that these and related questions will be solved within the next few years, through efforts of several laboratories.
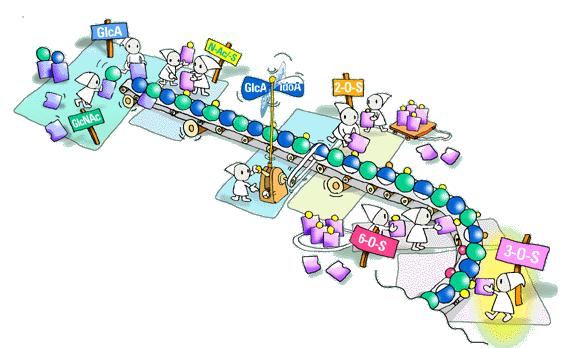
QIt does not seem easy to analyze the fine structure of HS chain. What methods or techniques should be developed to study fine structures of HS in the future?
HS research will most likely continue along two main lines. One is the biosynthetic mechanism that we already talked about. The other involves precise sequence analysis of HS domains, including saccharide regions implicated in specific protein binding. The sequencing problem is a difficult one, and differs in several regards from those encountered in protein or polynucleotide analysis. One difficulty, of course, is the heterogeneous nature of HS populations; one will have to isolate defined, minimally modified sequences with a given functional role and apply those to sequencing. As for methods, there are several alternatives, including mass spectrometry (which may be hampered by the abundance of isomeric disaccharide units), NMR and stepwise enzymatic degradation. Again, I am sure that we will see quite a dramatic development in this area within the next few years. The obvious goal is to combine precise HS sequence information with an understanding of the underlying biosynthetic regulatory mechanisms.