Hiroshi Nakada
Kyoto Sangyo University, Faculty of Engineering
Mucins are major glycoprotein components of mucus, covering the luminal surfaces of epithelial respiratory, gastrointestinal and reproductive tracts. Mucins contain numerous carbohydrate chains attached to the polypeptide (core protein, apomucin) mainly through O-glycosidic linkage. The core proteins of mucins are encoded by different mucin genes (MUC genes). Aberrations in the cell surface carbohydrates including mucins have been regarded as a universal characteristic of the malignant transformation of cells. These alterations are considered to be relevant to the abnormal behavior of cancer cells, such as altered cell adhesion or metastasis and to the avoidance of immunological defense.
Core protein
Recently, a number of mucin core proteins have been cloned and sequenced fully or partially. Most of them contain multiple tandem repeat domains. These tandem repeat domains are rich in serine and threonine residues and correspond to the most O-glycosylated part of the mature mucins. MUC1 protein, epithelial membrane associated glycoprotein (Episialin), is the first mucin to be characterized in detail. Normally this glycoprotein is present at the apical side of the cells in breast and milk fat globules. It is also expressed in pancreas, kidney and several glandular epithelial cells. The localization and interaction with the cytoskeleton suggest that MUC1 protein plays a role in determining epithelial cell polarity. Since the molecule sticks out from the cell surface, it can prevent cellular adhesion by masking adhesion molecules. MUC1 protein has an apparent molecular mass of more than 300 kDa, and 50% of the molecular mass consists of O-glycans. This protein has a small N-terminal region, a central region composed of the tandem repeats of 20 amino acids containing 25% hydroxylated amino acids, a transmembrane region and a short cytoplasmic C-terminal region. The polymorphism is produced by variable numbers of the tandem repeats. The MUC1 gene is located on the q21-q24 region of chromosome 1. Reactivity of antibodies directed against the tandem repeat increases remarkably in various tumors due to differences in glycosylation of MUC1 protein. The epitope is exposing peptide which is normally glycosylated. Cytotoxic T lymphocytes isolated from patients with breast carcinoma recognize the underglycosylated peptide of MUC1 protein. Core proteins of several secretory mucins have been cloned. One of these, MUC2 protein, which is expressed in intestine, has been studied extensively. The gene encoding for the MUC2 protein is mapped to the p15 band of chromosome 11. The complete MUC2 cDNA corresponds to an apomucin containing more than 5000 amino acids. The protein consists of two regions rich in serine and threonine which are potential O-glycosylation sites: a domain of irregular serine-, threonine- and proline-rich repeats and a large domain containing the tandem repeats of 23 amino acids. In humans, at least nine different mucin genes encode epithelial mucins. Most mucins contain tandem repeats and are polymorphic due to variable numbers of the tandem repeats and their transcripts are often heterogeneous. With regard to the gene expression of mucins with malignant transformation, the current data are difficult to generalize. Both up-regulation and down-regulation of mucin gene expression have been reported for malignant cells.
Carbohydrate chain
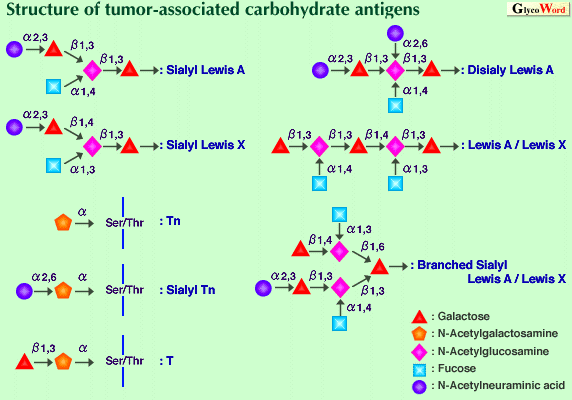
Most mucin carbohydrate chains are attached to the core protein through N-acetylgalactosamine via a-O-glycosidic linkage to the hydroxyl oxygen of serine or threonine. In addition to N-acetylgalactosamine, fucose, galactose, N-acetylglucosamine and sialic acid are commonly found in mucins. In O-glycans, three domains can be distinguished: a core, a backbone and a peripheral region. The core region of the O-glycans consists of the linkage GalNAc and the sugars directly attached to it. The GalNAc substituted on the hydroxyl of C3 either by a Gal (b1-3) or a GlcNAc (b1-3) constitute core 1 and core 3 structure, respectively. Attachment of GlcNAc by (b1-6) linkage to core 1 and core 3 produces core 2 and core 4, respectively. The GalNAc is also substituted by a GalNAc (a1-3) (core 5) or by a GlcNAc (b1-6) (core 6). Mucins can have any combination of the different core structures. Backbone regions are composed of disaccharides : Gal (b1-3) GlcNAc (type 1) and Gal (b1-4) GlcNAc (type 2). The type 1 and type 2 structures can be further branched at a Gal residue by (b1-6) and (b1-3) linked GlcNAc residues. The backbone chains are terminated by peripheral sugar, i.e. a-glycosidically linked galactose, N-acetylgalactosamine, fucose or sialic acid. A large number of monoclonal antibodies directed against carbohydrate antigens have been produced. These monoclonal antibodies were extensively used for characterization of tumor-associated carbohydrate antigens. The antigens comprise peripheral structures representing blood group antigens as well as core structures such as Tn and T and their sialylated counterparts, sialyl Tn and sialyl T, as shown in the Figure. The tumor-associated carbohydrate antigens characterized in detail both structurally and functionally are sialylated Lewis A and its isomer sialylated Lewis X. These structures are found in glycolipids and mucins of tumor cell membranes and in mucins circulating in the serum of cancer patients. It is well known that they are ligands for a selectin family which is assumed to play a role in cancer metastasis. Many related or more complex carbohydrate antigens such as disialyl Lewis A, a hybrid-type structure, i.e. Lewis A and Lewis X, sialyl Lewis A and Lewis X and so on, have been reported. These structures have different affinities to selectins.
In contrast to these type 1 and/or type 2 chain epitopes, antigenicity is produced by shortening of carbohydrate chains. These unmasked epitopes are found on the plasma membrane of most epithelial cell lines derived from lung, breast, colon and pancreas tumors. The clustered structure of Tn and sialyl Tn antigens is essential for potent antigenicity. Although the role of sialyl Lewis A, X and related antigens as ligands for selectins is well accepted, the functional role of incompletely synthesized carbohydrate chains and their sialylated carbohydrate chains remains unclear. Many clinical studies show a clear relationship between the presence of these antigens and long-term survival rates.
Mucin like membrane protein
The term mucin has recently expanded to include membrane-bound mucin-like molecules with highly O-glycosylated domains, such as leukosialin and GlyCAM-1. PSGL-1, which is expressed on leukocytes, has ligand activity for selectins. Both sulfated tyrosine and carbohydrate chains are necessary for high affinity binding to P-selectin. GlyCAM-1, CD34 and MAdCAM-1 are also mucin-like molecules presenting a carbohydrate ligand for L-selectin.