
Professor Naoyuki Taniguchi
Professor Taniguchi graduated from the Hokkaido University School of Medicine in 1967, and completed the doctoral course of medicine at the Hokkaido University School of Medicine in 1972. He was then appointed an assistant professor at the Hokkaido University School of Medicine in 1975 and appointed a visiting associate professor, Department of Biochemistry Cornell University Medical College, New York at the laboratory of Dr. Alton Meister between 1976 and 1977. He was appointed an associate professor, Graduate School of Environmental Science, Hokkaido University in 1977 and later appointed an associate professor, Biochemistry Laboratory Cancer Institute, Hokkaido University School of Medicine in 1980. He was appointed as a Professor and Chairman, Department of Biochemistry, Osaka University Medical School in 1986. He received a Grant in Aid for Scientific Research on Priority Areas entitled "Sugar remodeling and cellular communications" from the Ministry of Education, Science Sports and Culture, Japan between 1998 and 2000. Organizer of the 1st International Symposium on Glycosyltransferases and Cellular Communications, 1997.
The meeting president for Japanese Biochemical Society in 2001. Honorary member of the American Society for Biochemistry and Molecular Biology. The secretary general for International Union of Biochemistry and Molecular Biology, 2006. Editorial board members: J. Biol. Chem., Glycobiology, Glycoconjugate J.,etc.
The day of complete nucleotide sequence determination of the human genome is near at hand, and there is an increasing expectation that the obtained data will be utilized for the clarification of disease-related genes and genome-based drug design. In the field of glycoscience, also, genes encoding a large number of glycosyltransferases involved in the synthesis of the sugar chains have been cloned, and a systematic analysis of their functions has been promoted. Under these circumstances, studies in glycoscience are being focused on examining the significance of various types of sugar chain structures and elucidating the mechanisms of biological regulation for carbohydrate synthesis.
In Japan, many studies on complex carbohydrates have long been accomplished by members of the research groups supported by the Ministry of Education, Science, Sports and Culture (MESSC), and excellent results have been produced. Currently, a project "Sugar remodeling and cellular communications", has been formed and tackling the subject. We interviewed Professor Naoyuki Taniguchi, a prominent researcher in this field, asked some questions about professor's research history, current matters of concern, and future study subjects in the glycoscience field.
QHow did you become involved in the study of sugar?
When I was a postgraduate student at Hokkaido University, I was first given a research project focusing on the chemistry of sulfur-containing compounds and began with the study of glutathione, a typical sulfur-containing compound. Glutathione is a low molecular compound that plays the most important role in oxidation/reduction. The isolation and purification of γ-glutamyl transpeptidase (γ-GTP), an enzyme that degrades glutathione, was the subject of my thesis. I then continued to study this subject under the guidance of Professor Meister Cornell University Medical College and Professor Makita of the Cancer Institute at Hokkaido University, and focused on the examination of cancer-associated changes of γ-GTP and its enzymatic mechanism.
QThe γ-GTP happened to be a glycoprotein, didn't it?
Although it has been demonstrated that there is heterogeneity in the sugar chain of purified γ-GTP isolated from fetal and cancer cells, we were, at that time, unable to analyze the sugar chain. Fortunately, Professor Kobata (Kobe University), who was interested in carbohydrate component of the γ-GTP, offered a cooperative study. During our collaboration, we discovered a bisecting GlcNAc structure, which was, I think, how our current study came about.
After moving to Osaka University, we developed a method for measuring the activity of GnT-III, an N-acetylglucosamine transferase (GnT) that produces bisecting GlcNAc, and from there our work progressed to enzyme purification, cDNA cloning, and gene cloning.
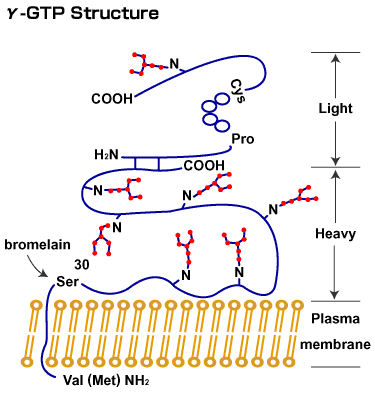
QHow did you expand the range of the study investigating the sugar chain of γ-GTP?
It has been clarified that, in rats, bisecting GlcNAc, which is almost negligible in the normal γ-GTP (which has complex type sugar chains), exists in a large quantity only in that isolated from hepatic cancer. With cancer, there is approximately a 100-fold increase in GnT-III activity in the liver. This enzyme does not exist in the normal liver, but exists in the greatest amount in the kidney. Therefore, purification of this enzyme was initiated using rat kidneys. We spent about 3 years on isolation and purification of this enzyme.
We have also succeeded in the gene cloning of glycosyltransferase. After GnT-III, we succeeded in cloning GnT-V. Unfortunately, this was accomplished by another group just 1 month earlier. However, we independently have succeeded in gene cloning after isolating the same enzyme from human tissue.
After this, we succeeded in the purification of α-1,6-fucosyltransferase (FucT), an enzyme that transfers a fucose to the linkage chitobiose of α-fetoprotein (AFP). AFP increases when hepatic cancer appears and is also detected in the blood when acute hepatitis or liver cirrhosis occurs. Therefore, AFP is, of course, useful as a tumor maker, but the accuracy of diagnosing cancer may only be about 65-70%.
On the other hand, AFP with α-1,6 fucose is fairly specific to hepatic cancer. Since we thought it interesting to examine the mechanism of α-1,6 fucosylation, we conducted gene cloning after the purification of 1,6 FucT from human gastric cancer cells and the porcine brain.
In cancer, abnormalities often occur at the branching site of the sugar chain. For this reason, we are interested in enzymes involved in the branching of the sugar chain. That is to say, branching is involved in all events of our interest.
For all studies hitherto mentioned, we did not conduct so-called "homology cloning" using EST. Rather, our strategy was to conduct gene cloning after isolating protein and examining the properties of it.
QMany GnT enzymes seem to be present, what is its significance?
Well, GnT-I knockout mouse is lethal. GnT-III, IV, V, or VI, which are involved in the formation of the branched sugar chain, have no mutual homology. In this sense, these are rather new genes. That is, these genes are not contained in every organism. For example, they are not contained in yeast but are found in some nematodes.
Glyco-genes are expressed in a manner specific to organs, tissues, or cells. Therefore, their activities may completely differ depending on the site of their expression, such as in cancer, in the kidney, or in the brain.
Since the β-1,6 Gn sugar chain is involved in cancer metastasis, GnT-V that synthesizes this sugar chain is considered to be a bad guy. However, GnT-III is regarded as a good guy since it inhibits the production of β-1,6 Gn by forming β-1,4 bisecting GlcNAc Here, it should be noted that in transgenic mice, in whom GnT-III is overexpressed, new diseases may appear such as fatty liver resulting from lipid accumulation. In this case, GnT-III is not a good guy. It is not yet known what happens when GnT-III is expressed in other organs, although there may be other findings that suggest that GnT-III inhibits the sensitivity to spleen NK cells, and that the overexpression of α-1,6 FucT leads to Wolman disease, a disease characterized by fat accumulation.
Therefore, I think there are considerable differences in the functions of glyco-genes depending on the organs or cells. The significance of their presence may also differ.
QWhat about the chromosomal localization of the genes for these enzymes or regulation of their expression?
Chromosomes for GnT-III, IV, V, and α-1,6 FucT are located on different chromosomes. It is possible that they may be related to some diseases, but at present, there is no evidence to support this. Their functions are completely different, probably because they are differentiated at a relatively early evolutionary stage.
Concerning glyco-genes in general, the mechanism of gene regulation is only partially known for GalT, at present. Unfortunately in GnT-III gene, also, the mechanism of regulation has not yet been clarified.
QTo what extent have the protein structures of glycosyltransferases been clarified?
The 3D structures have gradually come to light, for example, those of α-1,3 GalT and β-1,4 GalT. And the x-ray crystallographic analysis of GnT-I has just been completed. For GnT-III and V, conducting the x-ray crystallographic analysis is difficult since they have sugars. For α-1,6 FucT, analysis may be possible because α-1,6 FucT has no sugars. Since the domain structure of the genes for glycosyltransferases is similar, domains such as acceptor recognition sites and donor recognition sites may be found one right after the other. Thus, there may be a great advance in the understanding of enzyme reaction. However, questions addressing the manner in which these enzymes are located in the cell or in the Golgi, what kind of cluster they form, and how they actually synthesize the sugar chain, may take some time to be answered.
QWhat about the functions of the formed sugar chains?
Sugar chains interacting with selectin have been successively identified. As for ligands that recognize glycoprotein, those specifically recognizing N-glycans, is very difficult to find, though there are, of course, some exceptions such as C-type lectin. The way to approach this problem may only be by attaching such specific sugar chains to solid phase materials in order to select proteins that bind to the sugar chain, or by making use of methods such as the 2-hybrid system.
At present, only a little is known about where lectins, which recognize sugar chains are present and their properties. For example, although the β-1,6 branched structure abundantly appears in metastatic cancer, how the cancer cells recognize it has not been clarified. We are thinking of carrying out studies in this area in the future.
QHow can we use gene knockout experiments?
The GnT-V gene has been successfully knocked out, and it has been shown that the defective mouse has less probability of developing cancer. Mice with defective GnT-V gene rarely developed cancers, and those injected with the GnT-V gene developed cancers. This means that GnT-V is similar to an oncogene. For GnT-III gene, though not many phenotypic changes are detected, it is considered that the resistance to chemical hepatocarcinogenesis appeared to increase if knockout is performed. This probably means that different situations may occur depending on the type of organ. In this sense, GnT-III can work both ways as a double-edged sword.
QIn recent years, many genes for glycosyltransferases have been cloned. What are your thoughts regarding the direction of carbohydrate research?
In the above mentioned research group investigating genes for glycosyltransferases (Saito Group), the cloning of many genes has been completed. The group was rewarded with excellent results in the field of homology cloning using a sialyl motif. This group focused their research on the isolation and characterization of genes for the sugar chain in any case. I think they have succeeded in the cloning of about 70% of the glyco-genes known throughout the world. However, research in the field of biology remains to be developed.
The target of our current group is to examine the individuals, cells, phenotypes after introduction of a gene into the cells, and the results of knockout animals. Also, we initially proposed to study specific intercellular ligands, sialyl Lewis x, midkine, growth factors, and interactions and signaling through proteoglycans.
There are already about 400 publications, and I think we have achieved good results concerning isolation, determination of functions, and identification of genes for carbohydrate synthesis. In a year-and-a-half, we will have more new discoveries.
QSeveral genes for the sugar chain are involved in its formation. This situation is complex, isn't it, unlike that for proteins? What is the meaning of the research being done on the sugar chain as compared to the research analyzing the human genome?
Actually, glyco-genes include almost all the genes that encode the enzymes for sugar chain decomposition or lectin, although we chiefly mention glycosyltransferase since our research, by chance, focuses on glycosyltransferase.
By 2003, it is expected that the identification of all human genes will be completed. However, understanding of the cell biology solely through gene clarification may be almost impossible. Clarification of the cause of disease or the provision of a means for selecting therapeutic methods based only on the results may not be achieved.
The human body and its cells are full of diversification. Data for only one gene is probably not sufficient for the discussion of disease.
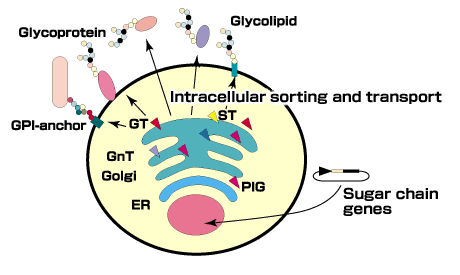
QIt has been considered that such diversity is inherent in gene function, which lead to more generalized concept of proteome. What is the significance of proteome from the viewpoint of glycoscience?
In the study of proteomes, proteins are identified using two-dimensional electrophoresis, and their properties and interactions discussed. One important point is that the diversity of proteome may be determined by sugars and phosphorylation. Therefore, the concept of a glycome corresponding to a proteome and of glycomics corresponding to proteomics could be forwarded. A more integrated form of glycobiology and glycotechnology is going to be called "glycomics". As a matter of fact, this thesis was already put forward 5 years ago by Dr.Vernon Reinhold, an American researcher. He proposed to apply the glycome concept to "post-translational modifications". For the science in which such technologies are utilized, the term "glycomics" may be applied.
When a certain gene is knocked out, a specific disease ensues and a causal relation can be established. However, in the case of the sugar chain, even if a glyco-gene is knocked out, it does not bring about a direct result but rather reflects the final figure brought about by the indirect knockout of other genes. The sugar chain modifies glycoprotein, glycolipid, and proteoglycan, which all affect matters in various steps, particularly the final step. Therefore, to reiterate this idea in another way, the study of glyco-genes may be useful because of the higher probability that there will be elucidation of events which exist in the down stream, in the place closest to where diseases are genarated. This is a rather direct manifestation. Nowadays, I often think we should place a little more emphasis on this way of thinking. Although the cause of disease is very complicated, what really controls the cause is the final stop. Therefore, it is not true that one gene has one function, but rather that a various combination of genes controls the function. The sugar chain may possibly define a function. This is why nowadays I conclude that this way of thinking is rather important. The concept of glycomes may cover this idea.
Presently, I am involved in the study of pig-to-human xenotransplantation. The problem is that there is an α-1,3 Gal epitope sugar chain on the cell surface in pigs and a natural antibody to this epitope exists in humans, which leads to the occurrence of hyperacute rejection. We do not have a technique to knockout pig genes that form this sugar chain yet. However, it was found that the synthesis of α-Gel epitope becomes negligible when bisecting GlcNAc is introduced into sugar chain using GnT-III. Therefore, I am currently studying the functional knockout of the epitope using this technique.
I would expect that the concept of glycomes will become very important as such studies or clarification of phenomena increase in number. In fact, glycome analysis using C. elegans has already been started overseas.