
Shuji Miyagawa
Dr. Miyagawa is a M.D., Ph.D., Associate Professor in Division of Organ Transplantation, Department of Regenerative Medicine, Osaka University Graduate School of Medicine. After graduate at Hokkaido University Medical School in 1981, he joined the First Department of Surgery, Osaka University Medical School, and was trained in general surgery and cardiovascular surgery during his residency. In addition to medical training, he became involved in research on the mechanism of discordant xenograft rejection, especially the activation of the alternative complement pathway. He received his Ph.D. degree at Osaka University Medical School in 1989. After serving as a fellow in the Division of Immunology and Organ Transplantation, Department of Surgery, University of Texas Medical School-Houston, USA, he resumed research in xenotransplantation in 1990. From 1994 on he has been involved in studies of basical glycobiology, especially glyco-antigen, aimed at reducing xenoantigenicity by means of enzymatic remodeling. His current research interests include the production of transgenic and knockout pigs.
Hobby-----skiing, hiking, watching baseball games

Ryota Shirakura
Dr. Shirakura is a M.D., Ph.D., Professor and Chairman of Division of Organ Transplantation, Department of Regenerative Medicine, Osaka University Graduate School of Medicine. After graduate at Osaka University Medical School in 1968, he joined the First Department of Surgery, Osaka University Medical School, and was trained in general surgery, pediatric and thoracic cardiovascular surgery during his residency. After worked for several years in different hospitals as research and clinical fellow, he backed to Osaka University Medical School and became involved in research on transplantation immunology, especially tissue typing. He received his Ph.D. degree in 1976. After serving as an honorary fellow in the Royal Society of London, Department of Cardiovascular surgery in June1980- March1981, he resumed the research on organ procurement and preservation. He became Associate Professor in 1990, and Professor and Chairman of same as above in 1994. His current research interests include xenotransplantation study, especially transgenic and knockout pigs.
Hobby----- golf, hiking, music

Naoyuki Taniguchi
Professor Taniguchi graduated from the Hokkaido University School of Medicine and obtained M.D. in 1967, and completed the doctoral course of medicine at the Graduate School of Hokkaido University and obtained Ph.D in 1972. He was then appointed an assistant professor at the Hokkaido University School of Medicine in 1975 and appointed a visiting associate professor, Department of Biochemistry Cornell University Medical College, New York at the laboratory of Dr. Alton Meister between 1976 and 1977. He was appointed an associate professor, Graduate School of Environmental Science, Hokkaido University in 1977 and later appointed an associate professor, Biochemistry Laboratory Cancer Institute, Hokkaido University School of Medicine in 1980 at the laboratory of Dr. Akira Makita. He was appointed as a Professor and Chairman, Department of Biochemistry, Osaka University Medical School in 1986. He received a Grant in Aid for Scientific Research on Priority Areas entitled "Sugar remodeling and cellular communications" from the Ministry of Education, Science Sports and Culture, Japan between 1998 and 2000. He is an honorary member for the American Society for Biochemistry and Molecular Biology. He will receive International Glycoconjugate Organization (IGO) Award, and Docteur Honoris Causa of the University Henri Poincaré in 2001. The president for the Annual Meeting of the 74th Japanese Biochemical Society in 2001. Editorial board members: J. Biol. Chem., Glycobiology, Glycoconjugate J., Biochem. Biophy. Res. Commun. (from March 2002).
The increasing problem of a worldwide shortage of donor organs has led to a revival of interest in xenotransplantation. Xenograftings can be classified as either discordant or concordant, such as pig to human or monkey to human, respectively, based on the severity and pattern of the graft rejection.
The pig represents an ideal animal for discordant xenografts for a variety of reasons, including anatomical, physiological and ethical considerations. Exposure of pig cells, tissues and organs to human blood, however, results in a so-called hyperacute rejection which is mediated by naturally occurring high-titer antibodies and complements in humans. The major xenoantigen responsible for this type of rejection is a single carbohydrate structure, the α-Gal epitope (Galα1-3Galβ1-4GlcNAc-R), expressed by most mammalian cells, including swine. The α-Gal epitope was first identified as a main component of rabbit erythrocyte glycolipids and was described as an internal type 1 chain, and later corrected to be a linear type 2 chain oligosaccharide. This antigen is the major antigen involved in pig to human xenotransplantation, which is synthesized by α1,3 galactosyltransferase (α1,3GT).1-3 Human and other Old World primates, however, are not able to synthesize this sugar structure because they have only a pseudogene due to a single nucleotide deletion at two separate positions which may serve to disrupt the translational reading frame. A reciprocal relationship exists between α-Gal epitope expression and the production of the anti-αGal antibody which is probably produced by constant antigenic stimulation by the α-Gal like epitopes which are located on the surface of normally occuring bacterial flora. Thus, humans acquire natural antibodies which account for as much as 1 % of the total circulating IgG, and which is also found in significant amounts as an IgM antibody.4,5
Trials designed to overcome hyperacute rejection by the expression of complement regulatory proteins, such as membrane cofactor protein (MCP; CD46), decay accelerating factor (DAF; CD55) and CD59 (MACIF, HRF20) on the graft has been shown to be quite effective.6 As the next step, trials, designed to overcome hyperacute rejection and acute vascular rejection, which is characterized by the presence of xenoreactive antibodies and the subsequent activation of graft endothelium, the activation of small amounts of complement, and incompatibility of the coagulation system, by modification of the glycosyltransferase(s) using gene technology are currently underway.
This strategy involves knocking out the α1,3GT, using an embryonic stem cell or somatic cell in conjunction with nuclear transplantation techniques. Gene targeting by homologous recombination in embryonic stem cells is not presently feasible still, since swine embryonic stem cells are not available for study. However, nuclear transplantation techniques from somatic cells to swine oocytes have been established at several institutes.7 Piglets in which one allele of the α1,3GT locus has been knocked out were produced by nuclear transfer, using pig fibroblasts, by two different groups.8,9
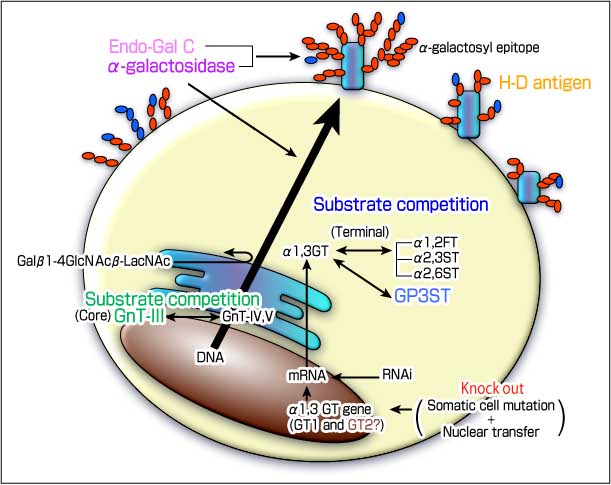
Fig.1 Various strategies, designed to downregulate the α-galactosyl epitope
The α-galactosyl epitope biosynthesized by α-1,3galactosyltransferase, is highly associated with hyperacute rejection in pig to human xenotransplantation. Variety of strategies have been pursued to reduce or eliminate this epitope from pig tissues.
Regarding with the K.O. of this gene, α1,3GT K.O. mice have been produced. Compared to wild mice, the cells and tissues of these mice bind to a lesser extent to the xenoantibody from human serum and are less sensitive to human complement. These mice, however, develop cataracts at 4-6 weeks of age.10,11
In terms of α1,3GT activity in organs, the mouse expresses a relatively lower α1,3GT activity than the pig in many organs. The differences are in the 10-fold range, except for the lung, based on our investigation.12 Galili et al also concluded that α-Gal epitope expression in pig organs is up to 500-fold higher than in mouse organs. Problems continue to exist regarding α1,3GT K.O. pigs, including severe deformity or lethality.
GT2 ( Fig. 1 shown by brown )
Sandrin et al reported, at the VI Congress of the International Xenotransplantation Association, that pig has two α1,3GT, that is GT1 and GT2. They clearly encode GT2 as a different gene from GT1, and the potency of GT2 for producing the α-Gal epitopes has been clearly demonstrated by staining with the Griffonia simplicifolia 1 isolectin (GSIB4 lectin), which bind to the α-Gal epitope.13 Concerning the existence of this gene in the pigs, further studies will be required.
A variety of strategies, designed to downregulate the α-Gal epitope have been reported. One of these involves controlling sugar chain biosynthesis using the β-D-mannoside β-1,4-N-acetylglucosaminyltransferase III (GnT-III) enzyme, which leads to a remodeling of the total antigenicity of the cell surface. Our previous studies showed a reduction in α-Gal epitope expression , in the case of pig endothelial cell transfectants. GnT-III catalyzes the branching of N-linked oligosaccharides to produce a bisecting N-acetylglucosamine (GlcNAc) residue. The attachment of a bisecting GlcNAc inhibits the further processing of oligosaccharides by other glycosyltransferases. That is, once a bisecting GlcNAc residue is added to the core mannose by GnT-III, the action of other competitive enzymes such as α-3-D-mannoside β-1,4-N-acetylglucosaminyltransferase IV (GnT-IV) and α-6-D-mannoside β-1,6-N-acetylglucosaminyltransferase V (GnT-V) are prevented from introducing any additional tri-antennary structures into the Golgi stack. As a result, it is likely that α-Gal epitope levels are decreased because the bisecting GlcNAc suppresses the further processing of complex-type sugar chains.
The disadvantage of this strategy is that GnT-III acts only on the N-linked sugar of a glycoprotein which is considered to have strong antigenicity. Therefore, other epitopes on O-linked sugar and glycolipids are basically unregulated by this enzyme.14,15
Another strategy for downregulating the α-Gal epitope is to take advantage of the enzymatic competition for terminal glycosylation between α1,3GT and other glycosyltransferases for the common acceptor substrate in the trans Golgi network by masking the Gal epitope by fucosylation or sialylation. Several glycosyltransferases, such as α1,2FT, α2,3 sialyltransferase (α2,3ST), as well as α2,6 sialyltransferase (α2,6ST) represent possibilities for achieving this. Among these, the gene transfection of α1,2FT has been reported to result in a drastic suppression of α-Gal.16 Sandrin, M.S. et al. reported an approximate 70 % reduction in α-Gal expression in a pig kidney fibroblast cell.17 Moreover, Sharma, A. et al demonstrated that this approach is effective in transfected CHO cells with α1,3GT and α1,2FT.18 Both reports also indicated that this approach for reducing the α-Gal is quite effective in the transgenic mouse. In addition, transgenic pigs which express α1,2FT have already been reported by several groups. However, as a disadvantage, Sepp, A. et al. demonstrated that LewisX expression was reduced to background levels, while the LewisY neoepitope was induced in the case of α1,2FT expressing pig cells.19
On the other hand, as we have previously reported, α2,3ST and α2,6ST dramatically suppresses the antigenicity of pig cells to human natural antibodies, more completely than the α1,2FT. In addition, we demonstrated that the combined transfer of GnT-III and α2,3ST appears to account for approximately 80% of the downregulation in xenoantigenicity.20
A novel sulfotransferase, glycoprotein-3-sulfotransferase (GP3ST) shows sulfotransferase activities toward oligosaccharides that contains nonreducing β-galactosides. GP3ST is able to act on both type 1 (Galβ1-3GlcNAc-R) and type 2 (Galβ1-4GlcNAc-R) chains with a similar efficiency. The functional feature of GP3ST to the α-gal epitope belongs to the terminal enzyme competition group, such as α1,2FT and α2,6ST. Because the substrate specificities of GP3ST are nearly the same as α2,6ST.
In our previous study, while the feature of the downregulation of xenoantigens, such as the α-Gal epitope, by GP3ST was very similar to those by α2,6ST, the degree of reduction of xenoantigen by GP3ST transfection is slightly milder than that by α2,6ST, about the same extent as α1,2FT. GP3ST transfection is effective in downregulating the α-Gal epitope in both glycoproteins and glycolipids.21,22
The elimination of the α-Gal epitope on the cell membrane by treatment with α-galactosidase represents a potentially effective approach, and the transgene of the cDNA is also effective. However, in spite of the drastic elimination of α-Gal in pig cells with the α-galactosidase gene, the transgenic mouse carring the α-galactosidase alone showed only a 15-25 % reduction in α-Gal content. Another study suggests that a combined transgenic approach using α-galactosidase and α1,2FT results in the continuous suppression of α-Gal in donor tissue.23
This enzyme was identified and purified from Clostridium perfringens culture media, has a strong activity for digesting α-Gal epitopes by cleaving the β-galactosidic linkage in the Galβ1-3Galβ1-4GlcNAc structure as evidenced, by an vitro assay and ex vivo perfusion as well. In addition, it is possible to inject Endo-Gal C into pigs and monkeys intravenously without serious side effects. This drug would be useful in eliminating the α-Gal epitope of the donor tissue as a temporary method. However, the production of a transgenic animal with this gene is not feasible at this moment.24
A combined approach with glycosyltransferases, such as α2,3ST and GnT-III, or α-galactosidase and α1,2FT, has already demonstrated the effective suppression of α-Gal. Therefore, a triple combination of GnT-III, fucosyltransferase or sialyltransferase, and α-galactosidase, might be the best approach to achieve the downregulation of xenoantigenisity at the present time. Needless to say, to achieve the best approach for overcoming hyperacute rejection, two or three complement regulatory proteins must be simultaneously added to the combined glycosyltransferases.20, 25
The Hanganutziu-Deicher (H-D) antigen, which contains N-glycolylneuraminic acid (NeuGc) is widely distributed in mammalian species, except for humans. The expression of NeuGc is controlled by cytidine monophospho-N-acetylneuraminic acid (CMP-NeuAc) hydroxylase activity. The absence of NeuGc in human glycoconjugates is due to a partial deletion in the gene that encodes CMP-NeuAc hydroxylase.26,27 Therefore, this epitope has the potential to become one of the largest epitopes in the pig to human xenotransplantaion after α1,3GT is knocked out.
Several strategies for reducing this antigen in pig cells are under consideration and include the K.O. of CMP-NeuAc hydroxylase gene.28 Fortunately, it is also possible for GnT-III to reduce the levels of the H-D antigen, because GnT-III does not act exclusively on an α-Gal.
On the other hand, Kobayashi et al. reported that no difference in the mean values of IgG and IgM antibody levels against NeuGc-GM3 was observed between healthy volunteers and patients with pig kidney perfusion 1 to 3 weeks previously or pig islet cell transplantation 2 months previously.29
Regarding transgenic pig that produced a glycosyltransferase, four groups have reported on a transgenic pig able to produce human α1,2FT. The first report was by Nagoya’s group. They actually produced transgenic pigs with α1,2FT, but could not maintain the transgenic line long enough for a clear analysis. The next report by Nextran, analyzed only a tail section of the transgenic pig. Another case was reported by Alexion Pharmaceuticals Inc. They used a H2Kb and a CMV promoter and obtained a good level of expression of the α1,2FT gene in most of the transgenic pig tissues, and analyzed modified cell surface carbohydrates, mainly fibroblasts. A 50 % reduction in the α-Gal epitopes of the PEC from the α1,2FT transgenic pigs was also shown, but they did not perform a transplantational experiment.30 The other report by Cowan, P.J. et al. described the combined transgenic pigs with CD55 (DAF)/ α1,2FT and CD55/CD59/ α1,2FT. However, α1,2FT expressions were relatively weak and did not significantly reduce the α-Gal epitopes, suggesting the importance of a combination of the complement regulatory proteins and the glycosyltransferases. 31
Concerning other glycosyltransferase, the transgenic pig carrying a high level of α2,3ST activity or α2,6ST is difficult to produce, judging from our previous experience.
On the other hand, GnT-III is expressed relatively easily in transgenic pigs. The expression of GnT-III in transgenic pigs clearly modifies the surface glycoantigen in most tissues and cells, in particular α-Gal, and confers resistance to human natural immunity. This approach leads to a remodeling of the total antigenicity, that is, not only the α-Gal but also H-D antigen and other unknown epitopes as well.(fig. 2) 12

Fig.2 Transgenic pigs with GnT-III
We have been successful in generating several lines of transgenic pigs that contain the human GnT-III gene. The overexpression of the GnT-III gene in pigs reduced their antigenicity to human natural antibodies, especially the α-galactosyl epitope as evidenced by several analysis.
The immunological barriers in the xenotransplantation field are becoming more well defined and promising strategies for addressing these are currently under development. At the present time, none of these approaches except for the knockout completely eliminates the expression of α-Gal. Pig endothelial cells express approximately 20 x 106 α-Gal epitopes. Therefore, Galili suggests that even a decrease in the α-Gal expression of 95 % may not suffice for preventing the rejection of a xenograft. Additionally, 10-30 % of the xeno-antigenicity to the human may remain on a swine cell membrane after the targeting knock out the α1,3GT.3
Further attempts to eliminate xenoantigenicity might be required in order to achieve successful clinical xenotransplantation.