
Dr. McCarthy received his B.A. from Susquehanna University in 1974. He obtained his Ph.D. in 1981 from Catholic University in Washington, D.C. His graduate laboratory training was at the National Institute of Dental Research, in Bethesda Maryland, under the mentorship of Dr. Sharon M. Wahl. His graduate research topic focused on acute and chronic inflammation. His specific research emphasis was on identifying important signaling pathways in activated macrophages that regulate the expression of collagenolytic matrix metalloproteinases. He accepted a postdoctoral associate position with Dr. Leo T. Furcht in 1981 at the University of Minnesota. His postdoctoral research addressed mechanisms by which the extracellular matrix proteins fibronectin and laminin promote the adhesion, migration and invasion of metastatic tumor cells. His current research focuses on the importance of cell surface proteoglycans and glycosaminoglycans in mediating tumor adhesion, growth and metastasis. He is currently a Full Professor of Laboratory Medicine and Pathology at the University of Minnesota in Minneapolis. Dr. McCarthy also serves as program leader for the Cancer Progression and Metastasis Research Program at the University of Minnesota Comprehensive Cancer Center.

Melanie A. Simpson: Dr. Simpson obtained her undergraduate degree in the Department of Biochemistry at the University of Minnesota, working on E.coli regulatory proteins in the laboratory of Dr. James Fuchs. After obtaining her B.S. in 1992, she pursued a graduate degree at the University of Minnesota in the Department of Biochemistry, Molecular Biology and Biophysics working with Dr. David Bernlohr. Her Ph.D. thesis work focused on the biophysical characterization of ligand binding properties of the Adipocyte Lipid-binding Protein, and studies of its role in adipocyte lipid metabolism using knockout mice. Upon completion of her Ph.D. in 1997, she became involved in hyaluronan research in the Department of Laboratory Medicine and Pathology with Dr. James McCarthy. Dr. Simpson finished her postdoctoral training in 2002 and accepted a tenure-track assistant professor position in the Department of Biochemistry at the University of Nebraska. Her current research interests are focused on understanding the molecular mechanisms that facilitate the synthesis and assembly of hyaluronan-rich matrices in tumors.
Prostate cancer is a major cause of cancer death in men, behind only lung and colorectal cancer in mortality. 1 Most prostate cancer is confined to the prostate at the time of diagnosis. As with other malignant tumors, progression is accompanied by increased invasiveness, synthesis of new vasculature, and eventual distant spread of the tumor. Locally confined prostate cancer is initially treated somewhat effectively by androgen ablation therapy. However, in many cases, tumor cells resume growth independently of androgens and become increasingly aggressive. Remote metastases occur most frequently in bone and lymph node, with some incidence also of visceral invasion. Bone metastasis is a major complicating clinical factor in prostate cancer patients, causing intense bone pain and decreased quality of life in patients with advanced disease. Defining the factors that contribute to tumor growth, vascularization and metastasis may facilitate better diagnosis of primary tumors and may lead to the development of novel cancer therapeutics.
Hyaluronan is a high molecular weight anionic polymer with mechanical and biological properties that influence tissue form and function. Hyaluronan-rich matrices are found in several normal tissues that are highly hydrated including vitreous humor, cartilage and the cumulus body of oocytes.2-5 Although hyaluronan is important for maintaining the hydration state of tissues, cushioning joints, and preserving cell free space, there are numerous studies that demonstrate its critical role as a biological stimulus. Hyaluronan is a required scaffold for normal neural crest cell migration, cardiac development, and prostate ductal formation. Hyaluronan has also been demonstrated as an adhesion substrate during wound healing and as an essential effector of cellular transformation from epithelial to mesenchymal phenotype during development.
Hyaluronan is synthesized in mammals by a family of three hyaluronan synthases: HAS1, HAS2 and HAS3.7-9 Although 50-70% identical at the amino acid level, gene sequences for the three human isozymes are localized to three distinct chromosomes: HAS1 (19q13.3-q13.4), HAS2 (8q24.12) and HAS 3 (16q.22.1). Secondary structure predictions and homology modeling indicate these integral membrane proteins have six transmembrane domains, and a seventh membrane associated domain.a,7 A large intracellular loop of about half the total molecular weight of the protein contains the enzyme active site and substrate binding domains. All three isozymes catalyze the same reaction: that is, successive alternating addition of glucuronic acid and N-acetylglucosamine from the respective UDP esterified sugar precursors, in a repeating disaccharide motif. Polymerization is concurrent with secretion of the growing chain to the extracellular space, such that its final size is not constrained by intracellular dimensions. Newly synthesized hyaluronan polymers range in average molecular mass from 1x105 to 1x107 Daltons.
a See articles by Weigel and by McDonald and Spicer in this series.
Hyaluronidase enzymes are responsible for degradation of the large hyaluronan polymers produced by hyaluronan synthases. Hyaluronidase-generated fragments of hyaluronan have different biological properties than high molecular weight hyaluronan polymers generated by hyaluronan synthases. Hyaluronidases are required for hyaluronan internalization and for the generation of small hyaluronan oligosaccharides that can stimulate neovascular development. In normal development and tissue function, increased hyaluronidase expression is usually associated with cells that are differentiated and therefore not rapidly dividing. There are five enzymes in the family of hyaluronidases, which have variable sequence similarity and active propertiesb. The loci encoding these enzymes are closely linked into two groups on chromosomes 3p21.3 and 7q31.3 b. Members of the family differ in expression both at the level of tissue specificity and subcellular localization. Post translational processing of the enzymes is also observed in a tissue specific fashion. Hyal1, the major hyaluronidase found in plasma, has an overall apparent molecular weight of 57 kD, 8 kD of which results from glycosylation of the protein core. By contrast, Hyal1 isolated from urine has a smaller molecular mass, resulting from proteolytic cleavage of the carboxyl terminal end of the proteinb. The significance of such tissue-specific differences in processing and/or expression of hyaluronidases is not entirely clear, however Hyal1 in urine has a higher specific activity than plasma Hyal1.
b See article by Stern and Csoka in this series.
Hyaluronan is highly anionic, bearing a formal negative charge on every disaccharide unit. As a large carbohydrate, hyaluronan is also very hydrophilic. Electrostatic repulsion between individual hyaluronan polymers, combined with extensive hydration of the polymers, confers a viscous, gel-like property to aqueous solutions of hyaluronan. The physiological consequence of high hyaluronan content within tissues is expansion of the tissue volume to create and maintain a loose matrix.10 This is a desirable property for cushioning of tissues such as joints and vital organs, which are dependent upon secreted high molecular weight hyaluronan for protection against physical damage. In addition to maintaining tissue hydration, the hyaluronan matrix can modulate diffusion of nutrients and small molecule effectors. Growth factors and cytokines, for example, may bind directly to components within such matrices and/or become locally concentrated as a result of limited diffusion. High tissue hyaluronan content may therefore also regulate responses of the tissue to extracellular stimuli.
Since hyaluronan is secreted as a free glycosaminoglycan, retention of a hyaluronan-rich extracellular matrix requires the presence of specific hyaluronan-binding proteoglycans and receptor proteins.10,11 Proteins that bind efficiently to the hyaluronan polymer often use a common structural motif known as a link protein homology domainc. The exact protein composition of hyaluronan-rich matrices varies in a tissue specific fashion, and can include hyaluronan-binding proteoglycans such as versican (described below) and aggrecan in conjunction with link proteins that stabilize their interaction with the matrix.
c See article by Day in this series.
Tumor cells may take advantage of hyaluronan-rich extracellular matrices, which are deficient in extensively crosslinked fibrous protein networks, to invade more easily into the surrounding tissues.4 Hyaluronan-induced tissue hydration physically creates spaces through which tumor cells may migrate and invade. Hyaluronan-rich matrices within the tumor-associated stroma are also infiltrated with newly forming blood vessels.12,13 These matrices, which may be formed and/or augmented in response to stimuli secreted by the tumor, are thought to provide an environment that is conducive to the survival and growth of tumor cells with increased vascularization of the growing tumor. Changes in hyaluronan content within primary tumors result from complex interactions between the carcinoma and the tumor associated stroma. Growth factors and cytokines produced by the carcinoma may stimulate fibroblasts embedded in the stroma to increase the production of hyaluronan and associated hyaluronan-binding matrix components, resulting in the assembly of hyaluronan-rich matrices in the vicinity of the expanding tumor. Accumulating hyaluronan stimulates growth, survival and invasion of the carcinomas, and hyaluronan fragments may contribute to angiogenesis. As tumors progress, the carcinoma cells may synthesize and retain individual hyaluronan-rich pericellular matrices, rendering them less dependent on hyaluronan produced by the associated stromal fibroblasts. Hyaluronan produced by the carcinoma may thereby act in an autocrine fashion to stimulate tumor growth and metastasis.
Since hyaluronan is involved in the regulation of normal developmental processes such as proliferation and migration, its overproduction often correlates to pathologies that are characterized by inappropriate cell division and motility. Importantly, elevated levels of circulating hyaluronan occur in patients with colon, breast, bladder and prostate cancers, and hyaluronan deposits are increased within the primary tumors. Depending on the tumor, these hyaluronan deposits are associated with the carcinoma and/or with the tumor-associated stromal cells. These observations emphasize a potentially important link between hyaluronan accumulation and tumor progression in humans.
Several studies examining hyaluronan content in human prostate cancer samples have correlated the intensity of hyaluronan staining within the tumor-associated stroma to the severity of the cancer. Patients exhibiting strong stromal hyaluronan staining had greater than a 50% overall decrease in 13 year survival compared to patients with low stromal hyaluronan.14 Detection of hyaluronan on tumor cells correlated weakly with perineural invasion of the tumor, an independent prognostic indicator. Although tumor cell hyaluronan staining did not correlate overall with prognosis, the patient group used in this study was not uniformly treated, making it difficult to precisely predict the relationship between hyaluronan and prognosis.14 A subsequent study by the same group addressed the prognostic value of stromal hyaluronan.15 Several ominous prognostic indicators such as PSA recurrence, seminal vesicle and perineural invasion, and presence of metastases correlated with strong staining of prostate stroma hyaluronan.15 Furthermore, hyaluronan staining has been found in locally invaded seminal vesicles and in lymph node metastases.16 Collectively, detection of elevated hyaluronan in prostate tumors appears to have prognostic value, and implicates hyaluronan in promoting both primary and metastatic tumor development.
A large fraction of tumor-associated hyaluronan is easily extracted from human prostate tumor samples, indicating that it is not extensively crosslinked by binding to aggregating proteoglycans or tissue-specific link proteins.16 Size exclusion analysis of this solubilized hyaluronan indicates a wide range in molecular weight with significant shifts to smaller oligomers, suggesting that tumor-associated hyaluronidases may be degrading this fraction. The prevalence of smaller hyaluronan fragments in tumor specimens, and their absence from normal prostate tissue, is consistent with a role for degraded hyaluronan in promoting tumor functions such as angiogenesis. Significant elevations in prostate hyaluronidase activity occur with advanced prostate cancer, relative to normal prostate or benign prostatic hyperplasia.17 Protein levels of Hyal1, a secreted hyaluronidase, have also been found elevated in patient tissue samples, correlating with advanced stage of the primary tumor and highest in lymph node metastases.16 Thus, hyaluronidase may function concurrently with hyaluronan synthase and hyaluronan-binding proteins to promote tumor progression.
Versican is one specific hyaluronan-binding proteoglycan that, like hyaluronan and Hyal1, accumulates within primary prostate cancer lesions in a manner that correlates to pathologic stage.18 The factors that lead to increased hyaluronan accumulation in the tumor are not entirely defined, but they may be related to increased levels of specific HAS isozymes and altered levels of specific hyaluronan-binding proteins that correlate to tumor stage. Increases in the amount of versican in the tumor associated stroma are produced in part by the effect of carcinoma derived TGF-β1 on the fibroblasts within the stromal compartment of the tumor.18 Additional studies documenting the altered composition of the hyaluronan rich extracellular matrix of primary tumors will be important for defining the mechanisms by which these matrices are stabilized or degraded to facilitate prostate cancer progression.
The cell surface retention and biological functions of hyaluronan rich matrices in prostate cancer progression also depend upon binding of hyaluronan by cell surface hyaluronan receptors and intracellular hyaluronan-binding proteins. CD44 and the receptor for hyaluronan mediated motility (RHAMM) are the two most studied cell surface receptors for hyaluronan, and both receptors have been implicated in the biology of tumor invasion and metastasis.d,19 The complexities of the ligand binding properties and signaling function of these two cell surface receptors have been rigorously discussed.10,19 Although a detailed review of the numerous studies on these receptors is beyond the scope of the current review, there are some salient features of each that may be directly relevant to prostate cancer.
d See articles by Turley and Harrison and by Knudsen and Knudsen in this series.
CD44 is a highly polymorphic integral membrane protein. Multiple CD44 isoforms are produced by alternative splicing and post-translational modifications. The binding of CD44 to hyaluronan affects cell adhesion and motility and, consequently, this receptor/ligand pair has been extensively studied in cell lines in the context of cell migration, growth and survival.19 CD44 can directly link to the cytoskeleton and stimulate a number of signal transduction cascades. In particular, CD44 has been reported to be tightly coupled to p185HER2/neu and c-src tyrosine kinases and shown to activate rho-family GTPases. CD44 also interacts with the ezrin/radixin/moesin family of cytoskeletal linker proteins. CD44 is thus positioned as an important mediator of cytoskeletal reorganization, cell adhesion and motility in many cell types. Not surprisingly, CD44 has been directly implicated in adhesion, motility and invasion of both normal and transformed cells, and expression of specific splice variants of CD44 has been associated with metastasis of certain tumor cell lines.20
While the importance of CD44 in promoting adhesion and motility has been well documented, its role in prostate cancer cells seems to be largely as a metastasis suppressor. CD44 is expressed on the glandular epithelium within normal prostates. However, CD44 expression is downregulated in human prostate cancer progression partly as a result of promoter methylation.14,21-23 Early studies examining patients undergoing radical prostatectomy indicated that decreased CD44 expression in prostate cancer cells is an independent poor prognostic factor.24 A more recent retrospective study confirmed the prognostic value of decreased CD44, showing a 50-60% decrease in overall 13 year survival of patients with low tumor CD44 relative to those with high CD44.14 Consistent with these data, studies in Dunning rats demonstrate that expression of the standard isoform of CD44 in a metastatic rat prostate cell line suppressed metastasis without inhibiting tumorigenicity.25 Therefore, CD44 does not seem to function in prostate tumor cells merely by promoting invasion and adhesion.
CD44 has been shown to facilitate internalization and degradation of extracellular hyaluronan, and may serve to maintain the hyaluronan matrix.26 High levels of CD44 within prostate may promote the degradation of hyaluronan and therefore the loss of cell surface CD44 in a tumor might contribute to the accumulation of tumor-associated hyaluronan. However, the metastasis suppressor function of CD44 in cell lines has been shown to be independent of the hyaluronan-binding activity of this protein, suggesting that there are additional factors contributing to suppression of malignant behavior in the Dunning model by CD44 [27].
Less is known about the importance of RHAMM in the biology of prostate tumor progression. In other cell types, RHAMM is reportedly both an intracellular and a cell surface protein.19,28,29 The mechanisms regulating its subcellular localization are not well understood. Like CD44, RHAMM is expressed as several isoforms that are produced by incompletely understood mechanisms involving alternative splicing and post translational processing. Surface RHAMM has been implicated in promoting the motility and invasion of a number of cell types by a hyaluronan-dependent mechanism. RHAMM ligation by hyaluronan has been linked to intracellular signaling pathways that activate c-src, focal adhesion kinase and MAP kinases.19 Intracellular RHAMM (also known as IHABP) has also been shown to associate with the actin cytoskeleton and with microtubules. Hence it may assemble signal transduction pathways by acting as a linker molecule. RHAMM and HAS2 are concurrently upregulated during the cell cycle, and intracellular hyaluronan co-localizes to the mitotic spindle.e,28,29 One possible hypothesis, therefore, is that the RHAMM/hyaluronan complex may facilitate proliferation by regulating transit through critical cell cycle checkpoints.19
e See article by Evanko and Wight in this series.
Additional hyaluronan-binding proteins have been reported, but their expression has not yet been correlated to prostate cancer. One example is LYVE-1, a link protein homologue first identified on lymphatic endothelial cells.30,31 Tumor-induced lymphatic growth is one postulated mechanism for lymph node metastasis in breast cancer. LYVE-1 expressed on the expanded lymphatic endothelium may recognize hyaluronan presented by malignant cells that have left the primary tumor. This interaction between tumor cells and the lymphatic conduits could facilitate lymph node colonization by metastatic tumor cells.
The importance of the interaction between hyaluronan and its cell surface receptors for tumorigenesis and metastasis has been consistently demonstrated by the effect of its disruption. Transfection of tumor cells with dominant negative forms of CD44 or RHAMM, for example, interferes with hyaluronan-binding to endogenous CD44 or RHAMM, resulting in decreased tumor growth or increased apoptosis.19,32 Hyaluronan fragments of 3-10 disaccharides compete with hyaluronan polymers for binding to CD44. Inhibiting CD44/hyaluronan ligation with these fragments reduces subcutaneous tumor growth 33 and anchorage independent growth through increased apoptosis of the inhibited cell population. Collectively, the effects of inhibited receptor ligation by hyaluronan polymer are consistent with an essential role for receptor/hyaluronan polymer interactions in survival of the tumor cells. Enhanced tumor cell survival would be predicted to promote tumor expansion and progression. An implication of enhanced survival could also be altered responsiveness to cytotoxic therapies as a result of hyaluronan overproduction.
Hyaluronan oligomers or other agents that disrupt hyaluronan/receptor interactions may therefore be useful as adjunct therapies in the treatment of certain malignant tumors in humans.4
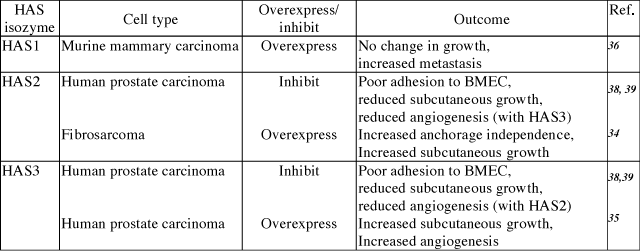
Numerous models have been used to correlate excess hyaluronan production by hyaluronan synthases with tumor cell invasion or metastasis (summarized in Table 1). For example, elevated hyaluronan resulting from overexpression of HAS2 in HT1080 human fibrosarcoma cells produces larger tumors in vivo and enhances growth in soft agar.34 Overexpression of HAS3 in the TSU prostate carcinoma cell line results in accelerated tumor growth.35 Mammary carcinoma cells selected for low hyaluronan synthesis exhibited significantly decreased lung colonization relative to high hyaluronan-synthesizing parental cell lines. This inhibitory effect on metastasis was completely reversed by restoring hyaluronan synthesis with transfection of HAS1.36 Our own recent experiments have demonstrated that inhibiting HAS activity in metastatic prostate carcinoma cells inhibits their adhesion to bone marrow endothelial cells as well as their ability to form tumors when injected into immunocompromised mice (Table 1 and discussed in more detail below).
Table. 1 Summary of hyaluronan synthase gene manipulation studies.
Studies in our laboratory have made use of human prostate tumor cell lines to identify factors important in prostate cancer progression.37-39 Our initial experiments were aimed at testing the hypothesis that prostate tumor cells, whose most frequent site of metastasis is bone, were able to colonize that tissue preferentially as a result of cell surface adhesion receptor distribution. We utilized prostate tumor cell lines of differing metastatic potential to perform a simple in vitro adhesion assay using cultured human bone marrow endothelial cell monolayers as a substrate. We found that non-metastatic LNCaP cells did not adhere well to bone marrow endothelial cells. In contrast, metastatic PC3 cells, and a variant of PC3 selected for enhanced metastatic propensity, PC3M-LN4, adhered rapidly and quantitatively to endothelial cells derived from bone marrow microvessels, but not those derived from large vessels, i.e. human umbilical vein endothelial cells (Fig.1). Furthermore, the rapid adhesion observed required prostate tumor cell surface hyaluronan, based on sensitivity to hyaluronidase digestion and competition with exogenous hyaluronan.37 Adhesion to bone marrow endothelial cells correlated roughly with metastatic potential of the prostate tumor cell lines, suggesting that the adhesive phenotype may be a feature of metastatic prostate cancer cells.
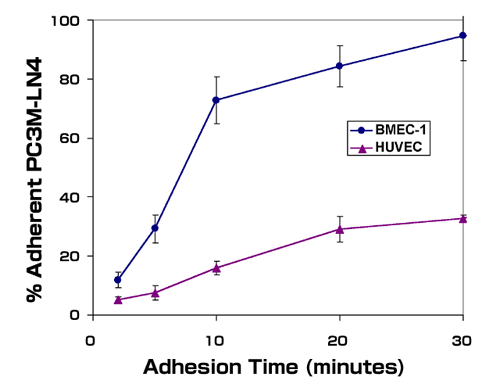
Fig. 1
Metastatic prostate tumor cells adhere preferentially to bone marrow endothelial cells. Single cell suspensions of metastatic PC3M-LN4 cells were labeled with calcein-AM and added to wells containing bone marrow endothelial cells (BMEC-1) or human umbilical vein endothelial cells (HUVEC). The wells were washed at the indicated time points, and adherent cells were then lysed and quantified by fluorescence intensity. Data represent the mean percentages of input cells from triplicate wells ± SEM.
Comparison of hyaluronan synthesis and cell surface retention by the prostate tumor cell lines also demonstrated a correlation between high hyaluronan production and metastatic potential.37 We used semi-quantitative RT-PCR to analyze and compare the expression levels of hyaluronan synthase in prostate tumor cell lines relative to normal prostate. HAS2 and HAS3 were found to be dramatically overexpressed in metastatic tumor cells, with no detectable HAS1. In contrast, the non-metastatic LNCaP cells expressed no HAS2 and very little HAS3, consistent with the absence of surface hyaluronan from these cells and poor adhesion to bone marrow endothelial cells. Similarly, we observed very low basal expression of HAS2 and HAS3 in normal prostate, which is consistent with histological reports in which low levels of hyaluronan are detected in normal human prostate tissue sections.16
To determine the impact of overproduced hyaluronan on tumor cells, we selected stable lines of PC3M-LN4 transfected with HAS2 and/or HAS3 partial cDNA in antisense orientation. Inhibition of either HAS isozyme alone by this method, relative to control transfectants, decreased hyaluronan synthesis and cell surface hyaluronan retention (Fig. 2), and adhesion to bone marrow endothelial cells (Fig. 3 and described in [39]). Greatest effects, not surprisingly, were manifest by simultaneous inhibition of HAS2 and HAS3. These results certainly demonstrate the efficacy of inhibiting hyaluronan synthases in limiting hyaluronan production and intercellular adhesion between prostate and bone cells. Furthermore, inhibition of this interaction by removal of hyaluronan from the surface of tumor cells suggests that a pericellular hyaluronan envelope borne by aggressive prostate tumor cells may promote bone metastasis.
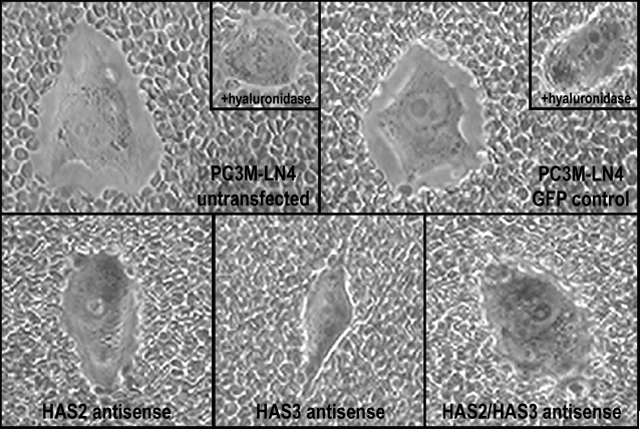
Fig. 2
Inhibition of hyaluronan synthase expression in PC3M-LN4 cells reduces cell surface hyaluronan retention. Cell surface hyaluronan was detected by particle exclusion. PC3M-LN4 untransfected, control vector transfected, HAS2 antisense transfected, HAS3 antisense transfected, or HAS2/HAS3 double antisense transfected cells were incubated with aggrecan (2 mg/ml) for 90 minutes. The aggrecan solution was removed and replaced with phenol red-free MEM containing 1x108 fixed sheep red blood cells. After a 15 minute incubation, cells were visualized by light microscopy at 400x magnification. Hyaluronan matrices surrounding the PC3M-LN4 untransfected and control cells are detected as a clear halo from which the erythrocytes are excluded. The hyaluronan content of these matrices was verified by hyaluronidase digestion prior to addition of aggrecan, following which the clear zones are no longer visible (inset panels).
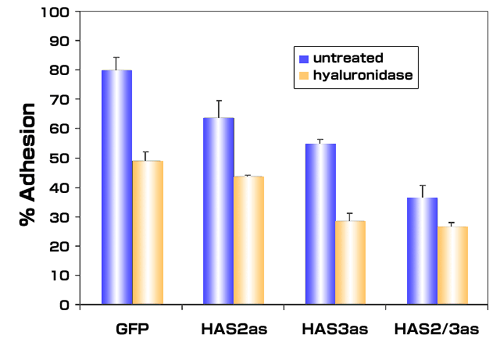
Fig. 3
Inhibiting expression of hyaluronan synthase expression in metastatic prostate tumor cells inhibits adhesion to bone marrow endothelial cells. Single cell suspensions of PC3M-LN4 cells stably transfected with either control vectors (GFP) or antisense vectors containing HAS2 antisense (HAS2as) or HAS3 antisense (HAS3as) were calcein-labeled. Cells treated with control medium or with 16 units/ml of Streptomyces hyaluronidase were then added to wells containing confluent bone marrow endothelial cell monolayers. After 10 minutes, the wells were washed to remove nonadherent and weakly adherent prostate tumor cells, and the adherent population was then solubilized and quantified by fluorescence. The mean adherent population was calculated as a percentage of input cells for each condition and plotted ± SEM.
The growth of metastatic and primary prostate tumors is accompanied by high levels of hyaluronan synthesis. We therefore evaluated the role of hyaluronan in tumor cell growth by subcutaneous injection of HAS antisense-inhibited PC3M-LN4 cells into immunocompromised mice.38 The results show a 75-80% reduction in the growth of tumors expressing either antisense HAS construct alone, or both in concert, compared to the vector control or untransfected cell lines (Fig. 4). Growth of the HAS antisense expressing cells in vitro was reduced in a fashion that correlated with decreased hyaluronan synthesis and retention, 39 but this inhibitory effect was diminished on more extended culture of these cells, which has also been observed in growing keratinocyte cultures expressing HAS antisense constructs. 40 The percentages of proliferating cells within the tumors did not vary, suggesting that altered growth properties alone do not fully account for reduced tumor sizes.
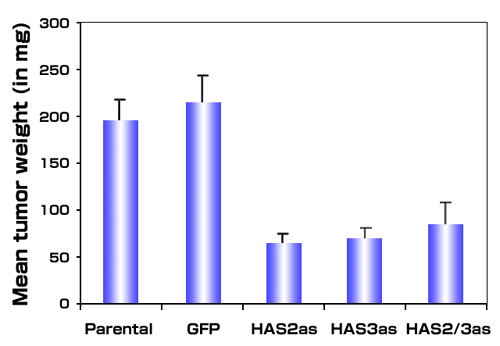
Fig. 4
Antisense inhibition of hyaluronan synthase expression inhibits tumor growth in vivo. Single cell suspensions of metastatic PC3M-LN4 cells or stable transfectants selected for expression of vectors alone (GFP), HAS2 antisense (HAS2as), HAS3 antisense (HAS3as) or both antisense vectors (HAS2/3as) were injected subcutaneously into immunocompromised mice. Tumors from 10 animals per condition were harvested and weighed after three weeks. Data are presented as mean tumor wet weight ± SEM.
Evaluation of the vasculature within tumors arising from control and HAS deficient tumor cells suggested that an additional explanation for decreased tumor growth kinetics is reduced blood vessel formation.38 Tumor angiogenesis was quantified by staining with antibodies for CD31, a cell surface blood vessel endothelial marker (Fig. 5). Decreased hyaluronan production by PC3M-LN4 cells led to a 70-90% reduction in the tumor vasculature. Since vessel density within tumor sections was diminished, inhibiting hyaluronan synthesis appears to alter the expression and/or function of angiogenic factors produced by the tumor. These results complement previous studies in which transfection of the TSU prostate carcinoma cell line with full length HAS3 leads to increased tumor growth and angiogenesis.35
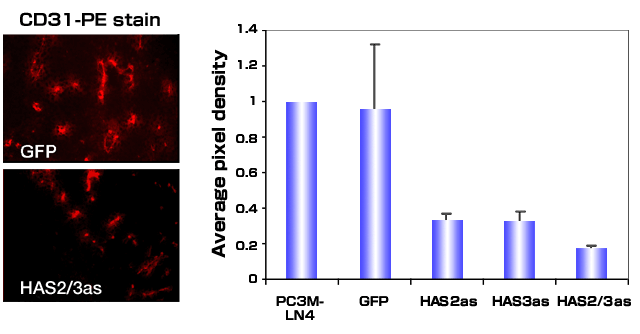
Fig. 5
Antisense inhibition of hyaluronan synthase expression inhibits tumor induced angiogenesis. Tumors grown subcutaneously in immunocompromised mice were harvested, frozen and sectioned. Tumor-associated blood vessels were visualized by fluorescence microscopy after staining sections with CD-31-phycoerythrin-conjugated antibody. A.) Representative sections from GFP control transfectants or HAS2/3as are shown. B.) Five random sections stained with conjugated antibody were digitally photographed, and images were processed using Adobe Photoshop. The average pixel density was determined for each transfectant, and the observed values were normalized to that observed for tumor sections containing the untransfected PC3M-LN4 cells.
Interestingly, the inhibitory effects on growth (Fig. 6) and angiogenesis (not shown) of HAS antisense expression in PC3M-LN4 cells were completely reversed by the addition of hyaluronan (average size 810 kDa) to the injection medium.38 This observation implies that promotion of tumor growth by hyaluronan occurs through stimulation of an early event following tumor injection and is complementary to results from Shuster, et al., in which hyaluronidase pretreatment of aggressive breast cancer cell lines dramatically reduced tumor growth.41 Early events stimulated by hyaluronan could include facilitated tumor cell survival by protection from apoptosis, or stimulation of an angiogenic response in the normal tissue surrounding the tumor. Regardless of the mechanism(s), the growing tumor is able to utilize hyaluronan derived from alternative sources. This observation implies that the increased hyaluronan detected in primary human prostate cancer stroma may originate either within the tumor cells or the stromal cells, and nonetheless influence tumor growth, local invasion, angiogenesis and metastasis.
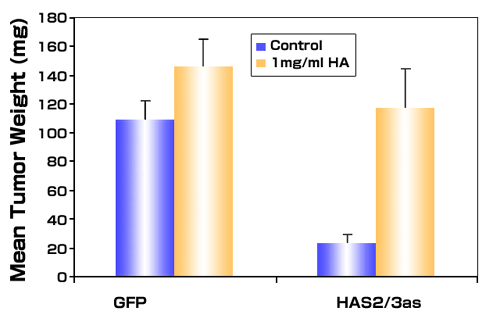
Fig. 6
Exogenous hyaluronan restores tumor growth of antisense-inhibited prostate tumor cells. Metastatic PC3M-LN4 cells stably transfected with either the vectors alone (GFP) or with double vectors containing antisense for HAS2 and HAS3 (HAS2/3as) were suspended in control culture medium or in medium containing 1mg/ml hyaluronan (average molecular weight of 810 kDa) and injected subcutaneously into immunocompromised mice. Tumors were harvested 3 weeks after injection. Data are presented as the mean wet weight of 7-9 tumors per condition ± SEM.
Human prostate cancer progression is accompanied by massive increases in hyaluronan and hyaluronidase content of the stromal matrix (Summarized in Fig. 7). Hyaluronan content of the matrix is modulated by the complex interplay of hyaluronan synthases, hyaluronidases, and hyaluronan-binding proteins. However, the mechanisms governing the expression and activity of hyaluronan metabolic enzymes and binding proteins are poorly understood. There is some evidence for genetic amplification of hyaluronan synthase in prostate cancer, but factors within the prostate tumor microenvironment are certainly an important consideration in the regulation of hyaluronan synthesis and degradation.
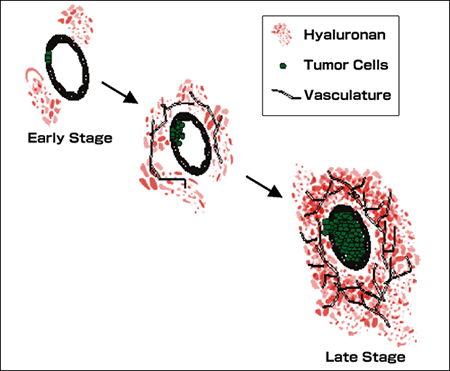
Fig. 7
Elevated hyaluronan in primary prostate tumors. Prostate cancer progression is associated with elevations in hyaluronan. The diagram depicts progression within the primary tumor of prostate cancer. Progression is associated with expansion of the glandular prostate carcinoma cells with a corresponding increase in the hyaluronan content of the tumor-associated stroma. The hyaluronan in advanced primary tumors is easily extracted and is diverse in molecular weight, containing smaller hyaluronan fragments that facilitate angiogenesis of the primary tumor. These smaller hyaluronan fragments are produced by the action of tumor-associated hyaluronidase which has also been associated with prostate tumor progression. These fragments, along with other tumor related factors, contribute to increased vascularization of the tumor that accompanies tumor growth and progression. The studies supporting this model are discussed in detail in the text.
Genetic aberrations that accompany prostate tumor progression are only now beginning to be identified. Such changes may modify the level of hyaluronan synthesis within tumors. Overrepresentation of segments of chromosome 8q.24 has been associated with prostate tumor progression.42 The loci present within this amplified region were partially characterized and found to include the coding sequence for HAS2.42 Analysis of prostate tumor cell lines showed a correlation between amplification of the HAS2 locus and elevated expression of HAS2 in the cells. Genetic overrepresentation within prostate tumor cells may represent an important mechanism by which HAS enzyme levels become increased in more advanced metastatic tumor cells.
Several growth factors and inflammatory cytokines have been shown to upregulate hyaluronan synthesis in cells and tissues.43-46 Most studies to date suggest that hyaluronan production is controlled primarily at the level of HAS transcription, though one recent study finds additional factors may be important for regulating HAS activity.43 Different HAS isozymes appear to be controlled by distinct regulatory elements, and observed differences are related to cell and tissue specific factors. TGFβ1, basic FGF and IGF-1, which are growth factors reported to be relevant to prostate cancer progression, have been shown to stimulate hyaluronan synthesis and/or HAS expression in various cell types. In these studies, HAS2 appears to be more transiently regulated than HAS3. HAS levels in certain tissues may also be important for regulating hyaluronan levels in primary and metastatic tumors. It will be important to determine the role of extracellular factors in stimulating upregulation of HAS isozymes specifically in prostate cells and prostate tumor stroma. The effect of such factors on hyaluronan degradation within the tumor is also undocumented and needs to be addressed.
Hyaluronidase expression in prostate tumor lines has also been evaluated. PC3 cells are reported to express Hyal1, Hyal2, and Hyal3, all at higher levels than normal prostate tissue.47 Hyal1 expression correlates directly with prostate tumor grade 16 and with the presence of smaller hyaluronan fragments, which are known angiogenic stimuli not normally found in prostate tissue. Several studies with transfected cells suggest that the concurrent overexpression of hyaluronan synthase and hyaluronidase is required for maximal tumor growth and metastasis. For example, Hyal1 overexpression in PC3M cells, which overexpress hyaluronan synthases, produced a slight increase in metastatic spread of these already highly metastatic cells.47 HAS2 overexpression in glioma cell lines that lack hyaluronidase actually reduced tumor growth, but all human gliomas tested expressed hyaluronidase.48 In a rat colon cancer model, HAS2 overexpression increased tumor growth, while Hyal1 decreased growth and yielded tumors with large necrotic centers.49 The interplay between hyaluronan synthesis and degradation in tumor progression remains an active research area.
Activated CD44 expression promotes clearance of hyaluronan in cartilage, and thereby regulates the level of chondrocyte hyaluronan matrix, important in maintaining joint integrity.26 It is likely that prostate tumor hyaluronan accumulation is similarly modulated by the level and distribution of CD44 and other hyaluronan-binding proteins in the tumor. These possibilities have not yet been investigated, but a thorough understanding of the transcriptional machinery regulating the level and integrity of tumor associated hyaluronan may yield new agents that can be used to disrupt hyaluronan matrix synthesis and inhibit tumor expansion and progression.
Understanding the mechanisms by which elevations in tumor-associated hyaluronan facilitate tumor progression is an important area of investigation in prostate cancer. There are clear associations between elevations in hyaluronan within the tumor stroma and unfavorable prognosis in patients.
Furthermore, our results and those of others suggest that the most advanced tumors may express hyaluronan synthases autonomously, and may condition their own microenvironment to facilitate growth, survival and angiogenesis of the primary tumor or its metastatic lesions. The presence of tumor cell surface hyaluronan may facilitate arrest of the tumor cells in bone marrow, and subsequently transduce signals for entry and sustained growth within that tissue. This is a clinically important feature of advanced disease in prostate cancer patients, and a potentially relevant target for therapeutic intervention.
Several key topics remain to be addressed. The effect of hyaluronan synthesis by the tumor, or the ability of tumors to stimulate hyaluronan synthesis in a paracrine fashion, may be important factors in facilitating growth and angiogenesis at the primary site. Furthermore, tumor associated hyaluronan may be important for stimulating local tumor spread, metastasis and/or growth within regional lymph nodes, or growth within bone and other secondary sites. The effects of hyaluronan on localized tumor spread and metastasis likely involve the interaction of hyaluronan polymers with tumor cell associated hyaluronan receptors that modify intracellular signal transduction pathways leading to enhanced survival, growth and motility. The use of defined human tumor cell lines is one important tool for helping to dissect these issues, however the use of transgenic animal models of prostate cancer will also be essential for studying the importance of dysregulated hyaluronan metabolism at different stages of prostate tumor progression. In humans, modifying HAS expression or activity may represent novel therapeutic approaches that can be used as adjunct therapy for primary tumors with the potential to become invasive and metastatic. It may also be possible to target HAS in metastases or locally recurring tumors that no longer respond to hormone ablation therapy. Such therapies may lead to the better control and management of metastatic tumors, especially within bones, where metastases cause significant clinical problems associated with a decreased quality of life.