
Tatsuya Yamazaki
Assistant Professor, Department of Microbiology and Immunology, Aichi Medical University, School of Medicine
2011 PhD, Department of Biological Science and Technology, Tokyo University of Science
2011-2012 Postdoctoral Researcher, Neurovirology Project, Tokyo Metropolitan Institute of Medical Science
2013-2016 Project Researcher, Division of Viral Infection, Department of Infectious Disease Control, International Research Center for Infectious Diseases, Institute of Medical Science, The University of Tokyo
2016- Current position
Dr. Yamazaki works on the development of new passive immunization using antibody gene-based injection.

Sachiko Akashi-Takamura
Professor and Chair, Department of Microbiology and Immunology, Aichi Medical University, School of Medicine
1992 MD, School of Medicine, Saga Medical University
1992-1994 Resident of Internal Medicine, Saga Medical University
1994-1995 Medical Doctor of Internal Medicine, Aso Iizuka Hospital
1995-1996 Medical Doctor of Respiratory Internal Medicine, Saga Medical University
1996-2000 Graduate Student, Department of Immunology, Saga Medical University
2000 PhD, Department of Immunology, Graduate School of Medicine, Saga Medical University
2000-2001 Assistant Professor, Department of Immunology, Saga Medical University
2001-2013 Assistant Professor, Division of Infectious Genetics, The Institute of Medical Science, The University of Tokyo
2014.1- Current position
Dr. Akashi-Takamura is working on an elucidation of immunological functions of serum protein MD-1 and complement control factor C4BP, and of an adjuvanticity in human skin treated with bee venom PLA2.
Sugar chains play important roles in antibody function. It is well known that the deletion of a sugar chain from IgG increases the cytotoxic activity. It was also reported that sugar chain deletion from IgE, which is the main topic of this review, decreases the binding level to FcεR, which is the high-affinity receptor of IgE. However, an enzyme that specifically digests the sugar chain on IgE and affects the function has not yet been found. We previously reported that commercial receptor-destroying enzyme (RDE) from Vibrio cholerae specifically modulated the construction of murine IgE and inactivated the function. We also revealed that RDE modulated sugar chains on IgE. In our current study, we are evaluating the effect against human IgE. We hope to contribute to the development of a new treatment for allergy by modulating the sugar chain to inactivate IgE.
IgE, which is well known as a major factor of type I allergy, was discovered by Ishizaka and colleagues approximately 50 years ago1,2. Even though IgE is present in very small levels in human blood and the half-life is very short compared with other isotypes 3,4, allergy caused by IgE can markedly decrease the quality of life (e.g., asthma) or even bring life to a critical state (e.g., anaphylaxis). Mast cells, which express FcεRI, a high-affinity receptor for IgE, also play a major role in type I allergy. After binding to FcεRI, IgE stably remains on mast cells for several weeks 3,4, and IgE-FcεRI complex bound with allergen then activates the mast cells via the cross-linking. Finally, inflammatory factors (e.g., cytokine and histamine) are released from the mast cells, followed by induction of allergenic symptoms 5,6.
Although free IgE is very unstable in human blood, it is stable on FcεRI. Moreover, IgE binding to FcεRI increases the expression on mast cells5. This condition is possible as IgE more easily binds to FcεRI, followed by increment of the allergic response (i.e., the sensitivity increases with very few allergens5). Thus, it is conceivable that the inhibition of IgE binding to FcεRI would be effective in the treatment of allergy. Therefore, humanized anti-human IgE (omalizumab), which is an antibody drug, was developed. Omalizumab is reported to be effective against severe asthma and chronic spontaneous urticaria 7.
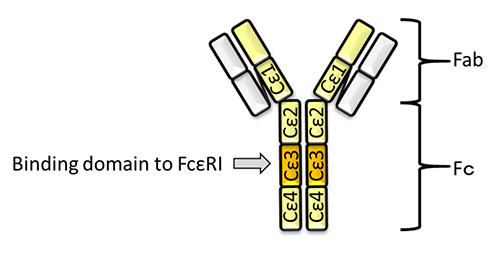
Here, we briefly introduce the mechanism of omalizumab for treatment for allergy. The constant region of IgE contains 4 domains, Cε1, Cε2, Cε3, and Cε4. Cε3 is the binding domain to FcεRI8 (Fig. 1). Omalizumab binds to Cε3 of IgE, followed by inhibition of the binding to FcεRI8. Mast cells are then restricted in their activation, even in their exposure to allergen, because of the lack of cross-linking of FcεRI. Therefore, omalizumab can suppress allergy. However, omalizumab cannot inhibit the IgE that has already bound to FcεRI on mast cells. This causes delay of several weeks to months in the effect of the treatment 8. Thus, new therapeutic strategy is required for allergy.
IgE is the most heavily glycosylated among isotypes. It has been reported that digestion of the sugar chains on IgE decreased the binding level to FcεRI, followed by restriction of allergic response 4,9. We also coincidentally found that commercial receptor-destroying enzyme (RDE) from Vibrio cholerae, which is usually used in neutralizing assay against influenza virus, specifically inhibited IgE binding to virus antigen 10. Further analysis revealed that RDE affected the sugar chain on IgE, and RDE-treated IgE decreased the induction level of allergy 11. Here, we focus on the relationship between IgE and the sugar chain, based on our own research and that of others, with intention to discuss the possibility of targeting the sugar chain on IgE as a new therapy for allergy.
Sugar chains are important for the function of antibodies. The structure of an antibody has a separate fragment antigen-binding (Fab) region and a fragment crystallizable (Fc) region, which vary according to their function 12 (Fig. 1). Fab contains the antigen-binding site, while Fc contains the domain that determines the specific function. Human IgGs (IgG1, IgG2, and IgG4) are glycosylated on a site of Cγ2 in Fc 13. This sugar chain is conserved at the 297th amino acid Asn (N297) of IgG, and affects the activity of antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and the binding level of Fc receptor (FcR) 14. On the other hand, IgG3, IgA (IgA1, IgA2), IgD, IgM, and IgE each have several glycosylation sites 13. IgE is most glycosylated at 7 sites (Murine IgE has 9 glycosylation sites 4). The sugar chain on IgE also affects the binding level of FcR similarly as for IgG.
Twenty years ago, human IgE treated with Peptide-N-glycosidase (PNG) from Flavobacterium meningosepticum was shown to decrease the binding level of FcεRI in vitro 9. Moreover, Shade et al. revealed that the chitobiose core of high-mannose sugars and some hybrid oligosaccharides from N-linked glycoproteins (also high mannose) at N394 in Cε3 are important to the binding to FcεRI. Mutant IgE (N394Q) and IgE treated with EndoF1, which can digest the high-mannose sugar chains, decreased the binding level to FcεRI 4. Those of murine IgE are also important for the binding level. The authors also indicated that the induction level of allergy was reduced with EndoF1-treated IgE in vivo.
However, it is difficult to directly inject an enzyme that digests the high-mannose sugar chains for treatment for allergy, because it could likely cause severe side effects. Therefore, it is important to consider an enzyme that more specifically digests the sugar chain on IgE, followed by the inactivation. We recently reported that commercial RDE from Vibrio cholerae specifically decreased the antigen-binding level of IgE and modulated the structure 11. We review the details in the following section.
Commercial RDE is prepared from Vibrio cholerae (Ogawa type 558) culture fluid. RDE, which is known to have protease and sialidase activity 15, has usually been used in the neutralizing titer assay against influenza virus, starting from a long time ago 15. Influenza virus binds to host cells via sialic acids. The specimens (e.g., serum) also contain sialic acids, which also bind the virus and cause non-specific inhibition of the infection. Therefore, the specimens usually need to be treated with RDE at 37°C overnight to avoid the non-specific inhibition.
Previously, we reported that neutralizing antibodies induced by antibody gene-based injection have prophylactic 16 and therapeutic 10 effects against influenza virus infection (also see our review about the details 17). In those studies, we constructed the plasmid expressing the neutralizing anti-hemagglutinin (HA) IgG, and also the plasmids expressing anti-HA IgM, anti-HA IgA, anti-HA IgD, or anti-HA IgE, the variable region of which was conserved 10. To confirm the neutralizing activity of each, we transfected human embryonic kidney (HEK) 293T cells with each plasmid and obtained the antibodies in the supernatant. We expected that all isotypes would have equal titer, because they have the same variable region containing antigen binding site. However, surprisingly, anti-HA IgE and anti-HA IgD could not neutralize the infection at all. Through further analysis, we found that RDE decreased the antigen-binding level of anti-HA IgE, but not of anti-HA IgG 11. This result suggests that RDE can inactivate IgE, and has possibility to have therapeutic effect for allergy. We therefore analyzed the function of RDE for the inactivation in more detail, as presented below.
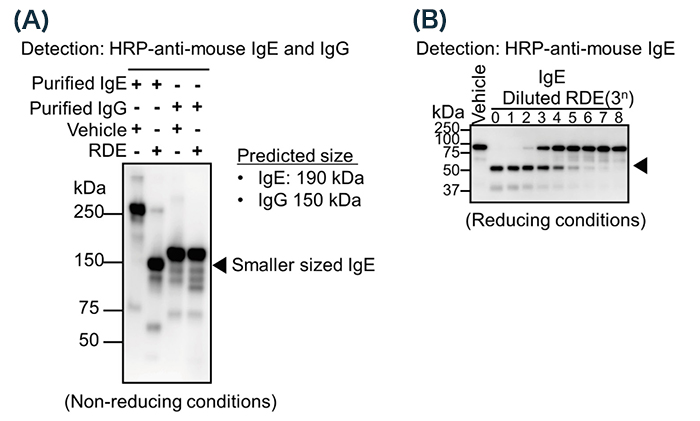
To confirm whether RDE can modify the structure of IgE, we treated purified IgE with RDE, and analyzed the band size by western blot analysis under non-reducing conditions. Although the predicted band size was around 190 kDa, the obtained size was over 250 kDa 11 (Fig. 2A, lane 1). This result suggests that IgE has a specific structure due to heavy modifications. Interestingly, the band size was much decreased by treatment with RDE, to around 150 kDa 11 (Fig. 2A, lane 2). As above, RDE has protease and sialidase activity, and the band size was not much different from predicted size (190 kDa). This result suggests that RDE affected the partial amino acid of IgE or sugar chain, followed by reducing the band size. By another western blot analysis under reducing conditions, we found that RDE reduced the band size of only a heavy chain (ε chain) (Fig. 2B), not a light chain 11. This result indicates that RDE affects ε chains, not light chains. On the other hand, purified IgG was not affected by treatment with RDE 11 (Fig. 2A, lanes 3, 4). These results showed that RDE specifically inactivated IgE.
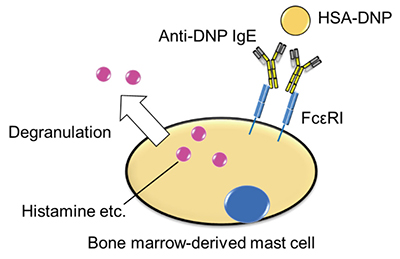
We confirmed that RDE modulates the construction of IgE. Next, we analyzed the induction level of allergy with RDE-treated IgE using bone marrow-derived mast cells (BMMCs). RDE-treated anti-dinitrophenol (DNP) IgE was bound to BMMC, followed by incubation with allergen (human serum albumin (HSA)-conjugated DNP). We then measured the degranulation level of histamine (Fig. 3), and found that the level with RDE-treated IgE was much reduced 11. We also found that RDE-treated IgE could not significantly bind to BMMCs 11. These results suggest that RDE modulates the structure of IgE, which then cannot bind to BMMCs and induce allergic responses.
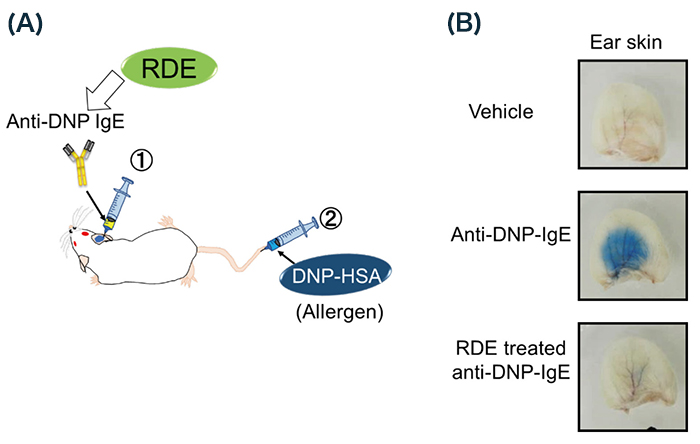
To confirm whether RDE-treated IgE also cannot induce allergy in vivo, we used passive cutaneous anaphylaxis (PCA) for analysis 4,18 (Fig. 4A). Briefly, Balb/c mice were injected with anti-DNP IgE via the ears, followed by injection of HSA-DNP containing Evans blue dye via tail vein. We then could easily recognize anaphylaxis in the area of the injection, because IgE-bound allergens increase blood vessel permeability as a symptom of allergic reaction (Fig. 4B). On the other hand, using RDE-treated IgE, we could not recognize anaphylaxis 11 (Fig. 4B). From these results, RDE-treated IgE abrogates the induction potency of allergy.
It is known that RDE has protease and sialidase activity, as mentioned above 15. To confirm whether the effect of RDE is an enzyme reaction or not, we heated RDE at 100°C followed by treatment of IgE. The RDE treatment did not reduce the band size of IgE 11. This result indicates that the effect of RDE is an enzyme reaction. However, we could not inactivate RDE by any protease inhibitor, and we also could not confirm whether purified sialidase from Arthrobacter ureafaciens modulated the structure of IgE similarly with RDE 11. These results suggested that the main function of RDE is neither protease nor sialidase activity.
According to previous reports, IgE treated with EndoF1 or PNG abrogates the binding to FcεRI 4,9. This indicates that modulation of sugar chain on IgE affects the binding level. Therefore, we analyzed the modulation of the sugar chain on IgE treated with RDE using lectin microarray 19, which is a type of microarray used for analysis of the details of glycan structures, involving the use of various lectins that are glycan-binding proteins, printed onto glass slides; our lectin microarray was printed with 95 kinds.
We treated two IgEs [purified commercial IgE (#1) and anti-DNP IgE (#2)] with RDE and analyzed the glycan structures using lectin microarray (Fig. 5A) to determine which lectins significantly reduce the binding level to IgE by treatment with RDE. First, the results showed that the binding level of the following lectins that bind to sialic acid was not significantly different: ADA (α2-6Sia), WGA (polySia), SNA (α2-6Sia), PVL (Sia), and LFA (Sia) 11. This result supports that of the experiment using purified sialidase from Arthrobacter ureafaciens. On the other hand, RDE significantly reduced the binding level of IgE to two lectins 11: Lycopersicon esculentum lectin (LEL), which binds to (GlcNAc)2–4 and (LacNAc)n 20, and Phaseolus vulgaris Leucoagglutinin (PHA-L), which binds to tri- and tetra-antennary complex oligosaccharides 21,22 (Fig. 5B, C). By lectin blotting with LEL and PHA-L, we also confirmed that RDE reduced the band signal of IgE 11. These results suggest that RDE mainly affects the sugar chains, which are recognized by LEL and PHA-L, on IgE. Interestingly, IgG could not be detected by lectin blotting with either LEL or PHA-L 11. This result suggests that RDE cannot modulate IgG, because IgG does not have the sugar chain recognized by LEL and PHA-L. Taken together, these results suggested that RDE specifically modulates the structure of IgE to affect the specific sugar chain, followed by inactivation of the function.
It is known that Vibrio cholerae has other types of glycosidase beside sialidase. One is amidase, which hydrolyzes monocarboxylic acid amide. During cell division, this enzyme plays an important role to digest peptidoglycan, a major component of the bacterial cell wall 23. Another is chitinases that digest chitin (poly-N-acetylglucosamine), which is also a major component of the bacterial cell wall and involved in the competency 24,25. To consider RDE in allergy treatment, we need to identify the glycosidase that digests the sugar chains recognized by LEL or PHA-L to inactivate IgE function. We also need to consider the safety, because it was reported that an enzyme such as tick chitinase causes the allergy 26.
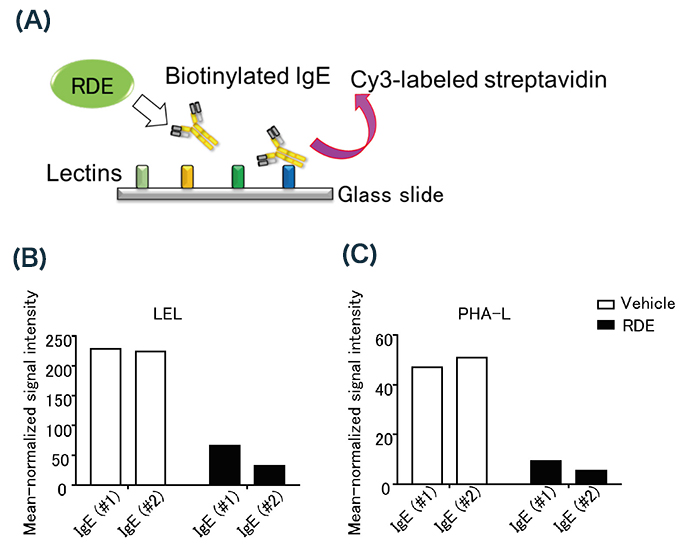
We are currently analyzing the effect of RDE against human IgE. Using western blot analysis, we have already confirmed that the band of human IgE was also much reduced by treatment with RDE (Unpublished data). Moreover, our research indicated that human IgE also binds to LEL and PHA-L by lectin blotting analysis (Unpublished data). We are now analyzing the binding level of RDE-treated human IgE to FcεRI and the induction level of allergy.
Sugar chains play very important roles in the function of antibodies. Removal of fucose from IgG increases ADCC induction via activation of NK cells 27. This kind of technique, involving removal of specific sugar chains, could contribute to the progress of the development of antibody drug. IgE, which is a heavy glycosylated antibody, plays one of the major roles in induction of allergy (e.g., asthma and pollen allergy). We hypothesize that the sugar chain on IgE can be a target of therapy for allergy. Therefore, we are identifying the factors involved in the inactivation of the function of IgE using RDE.
We thank Dr. Hiroaki Tateno and colleagues (National Institute of Advanced Industrial Science and Technology) for their performance of lectin microarray analysis, and also thank Dr. Teruko Imai (Kumamoto University) for discussion regarding inhibition of RDE with protease inhibitors.