
Yoshihiro Kamada
Current position: Associate Professor, Department of Molecular Biochemistry and Clinical Investigation, Osaka University Graduate School of Medicine
Academic degree: Doctor of Medicine
1995: Graduated from Osaka University Faculty of Medicine. Clinical internship in general internal medicine focusing on gastroenterology. Subsequently conducted non-clinical and clinical research on liver disease: from 1999, Department of Internal Medicine and Molecular Science, Osaka University (Professor Yuji Matsuzawa) and from 2005, Department of Gastroenterology and Hepatology, Osaka University (Professors Norio Hayashi and Tetsuo Takehara). From 2011 to present: Began research incorporating glycobiological techniques at the Department of Molecular Biochemistry and Clinical Investigation (Professor Eiji Miyoshi).

Eiji Miyoshi
Eiji Miyoshi MD and PhD, Professor and Chairman, Department of Molecular Biochemistry and Clinical Investigation, Osaka University Graduate School of Medicine
History: I graduated from Osaka University Graduate School of Medicine in 1986. After clinical training of Gastroenterology and Hepatology, I have been studying glycobiology. I became a professor in 2007 and was a dean of Osaka University Allied of Health Science in 2014-2015. I have been a director of the Center for Borderless Design of Medicine in Osaka University.
Research Interests: Research directed at understanding the biological functions of sugar chains at the cellular and molecular level and clinical investigation to many human disease including cancer, inflammation, DM and liver disease.
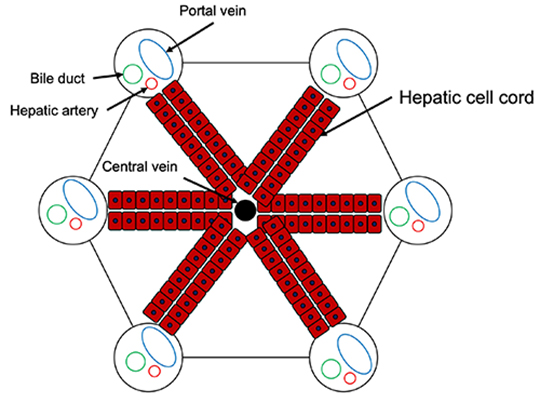
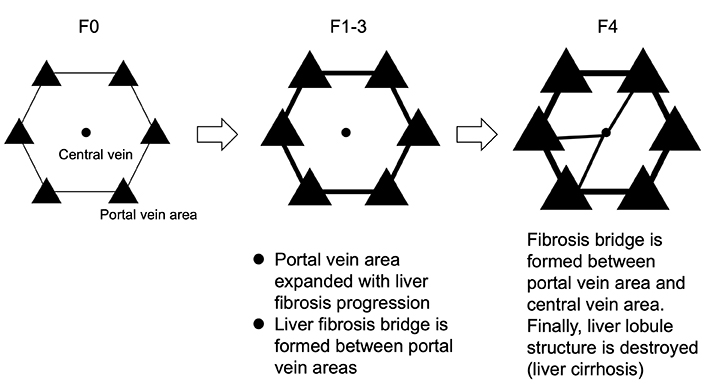
The normal liver has a hepatic lobule structure based on the arrangement of hepatocytes, and a hexagonal columnar structure around the central vein (Fig. 1(A)). There is interlobular connective tissue between hepatic lobules, and in fibrosis of the liver this region is expanded, and liver cells are killed and replaced by fibrotic tissue due to liver damage (Fig. 1(B)). Pathologically, liver fibrosis is classified into 5 stages, from F0 to F4 (F indicates fibrosis). In stage F4, normal lobule structure is completely destroyed and pseudo-hepatic lobules are formed 1. The pathophysiology of F4, which is the last stage of liver damage, corresponds to liver cirrhosis as described in the Introduction section. Even if liver fibrosis has progressed to F4, a state in which the liver function is relatively maintained is called “compensatory cirrhosis.” In contrast, a pathological condition accompanied by clinical symptoms such as ascites and hepatic encephalopathy is called “decompensated liver cirrhosis.” Regarding the pathology of liver cirrhosis, the Child classification, which includes biochemical tests and clinical symptoms, is well-known and is classified into three stages, from A to C.
Fibrosis is generally understood as a biological response following inflammation 2. Sudden fibrosis in normal liver is unlikely. Normally, when hepatocytes are damaged by hepatitis virus and/or alcohol, various immune cells infiltrate the liver's Gleason tree and cause various immune reactions. At this time, Kupffer cells (macrophages present in the liver) and hepatic stellate cells (HSCs) present in the liver sinusoids are activated. HSCs, also called Ito cells, are known as fat storing cells that store vitamin A. In the normal liver, HSCs are in the quiescent state, but cytokines and growth factors associated with inflammation induce the activation of HSCs. Transforming growth factor β (TGFβ) is the most important growth factor in liver fibrosis. Furthermore, a positive feedback reaction occurs in which HSCs are activated by TGFβ. The action of TGFβ induces collagen production from hepatocytes and forms fibrotic tissue. A protease that suppresses the fibrosis reaction is produced, and it determines whether fibrosis will progress greatly or less so, depending on the balance with the action of TGFβ and matrix metalloproteases.
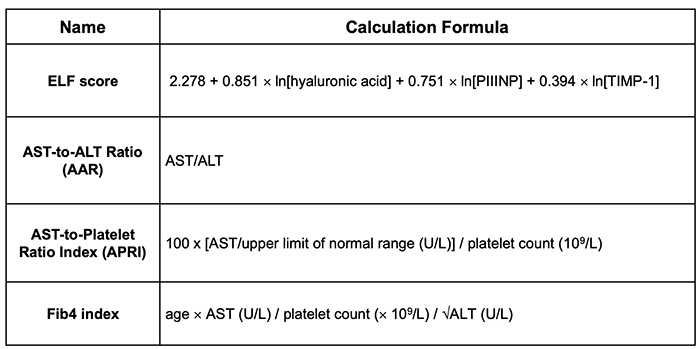
If TGFβ could be used as a liver fibrosis biomarker, assessment of liver fibrosis would be made easier. In general, cytokines and growth factors have short half-lives, and the measurement system itself is not stable. For this reason, hyaluronic acid, type IV collagen 7S, platelet numbers, Fib4 index, etc. are used as liver fibrosis markers clinically. When portal vein pressure increases with fibrosis of the liver, the spleen is swelled and platelets within it are destroyed. Platelet counts further decrease with the worsened function of hepatocytes in liver cirrhosis that produce thrombopoietin. The Fib4 index can be obtained from a simple formula using age, platelet count, and ALT proposed by Sterling et al. in clinical study against chronic hepatitis type C patients 3. Because of its simplicity, the Fib4 index is often used in daily clinical settings. In nonalcoholic fatty liver disease (NAFLD), the cutoff value of Fib4 index is different from that of chronic hepatitis type C 4. Enhanced Liver Fibrosis (ELF) score 5, AST-to-ALT ratio (AAR) 6, and AST-to-platelet count ratio index (APRI) 7, are often used for the assessment of liver fibrosis in chronic liver diseases (Table 1). In clinical settings, not only blood markers, but also abdominal echography and MR examination are often used in combination. Especially, elastography using ultrasound or MRI is used for the assessment of degree of liver fibrosis in clinical settings 8, 9.
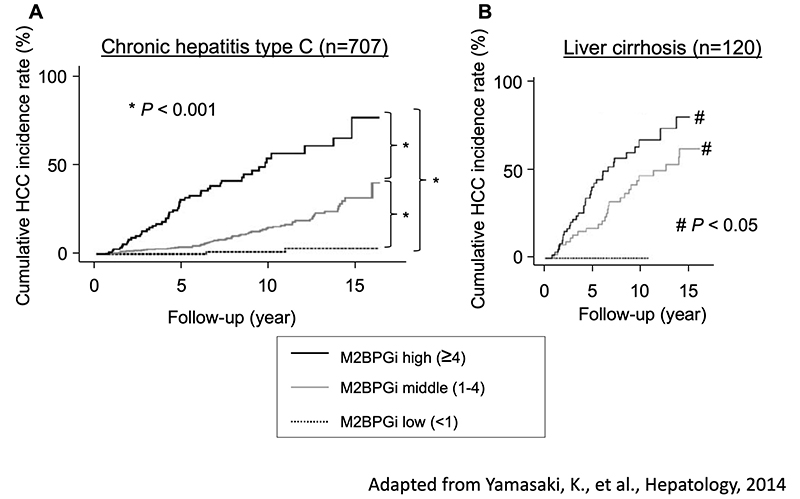
Mac2-binding protein glycosylation isomer (M2BPGi) is widely used as a liver fibrosis glyco-biomarker in Japan. M2BPGi is a glycan biomarker discovered by Prof. Hisashi Narimatsu of the National Institute of Advanced Industrial Science and Technology, and was listed by the Japanese National Health Insurance system as a clinical test in a high-throughput assay system using Sysmex's HISCL (high-sensitivity chemiluminescence enzyme immunoassay). M2BPGi was developed in NEDO: Glycan Function Utilization Technology Development Project in 2008-13. In search of cancer glyco-biomarkers from cultured cell lines, Mac2-binding protein was identified by glycoproteomics. Wisteria floribunda agglutinin (WFA) was found to be a lectin that shows low binding to other serum glycoprotein (low noise). The measurement principle is based on lectin-antibody ELISA 10. In particular, in chronic hepatitis C, M2BPGi increases stepwise as F1-F4 and liver fibrosis progress. Serum M2BPGi levels are dramatically increased at stage F4. Interestingly, even with the same F4 condition of liver cirrhosis, cases with high M2BPGi levels have a higher incidence of liver cancer (Fig. 2) 11. There is an opinion that M2BPGi is not a “pure” hepatic fibrosis biomarker. Hepatitis C virus (HCV) has been disappearing rapidly since oral direct-acting antivirals (DAAs) against HCV were developed in recent years, and M2BPGi levels have decreased rapidly in spite of the remaining liver fibrosis. M2BPGi should also detect the grade of liver inflammation. The weak point of M2BPGi is that the numerical values differ greatly in chronic hepatitis other than chronic hepatitis C.
We believe that Mac-2bp is associated with immuno-reaction of liver fibrosis 12-15.For example, in the case of non-alcoholic steatohepatitis (NASH), rather than M2BPGi, measuring Mac-2 binding protein (Mac-2bp) protein itself is a quick way to distinguish NASH from NAFLD 16. Serum Mac-2bp values in 510 liver biopsies from NAFLD patients were closely associated with liver fibrosis (Fig. 3) 17. Surprisingly, about 25% of patients with fatty liver who visited a medical dock had equivalent of NASH F3 and/or F4. However, in the study of Figure. 3, since there were few F4 cases, M2BPGi may have been more suitable if the number of F4 cases had been higher.
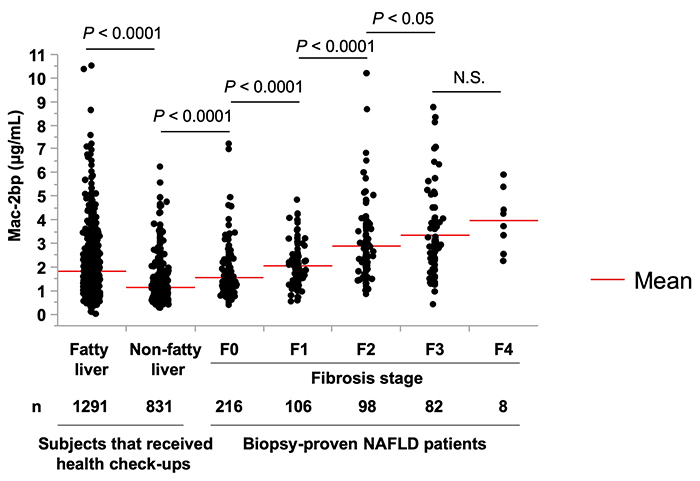
Compared to the liver development of cancer biomarkers, the reason why the fibrosis marker of the liver was found in the NEDO project may be that fibrosis is a pathological condition that occurs in the entire 1.5 kg liver. We should also objectively evaluate which is the better biomarker, Mac-2bp or M2BPGi. Immunohistochemical staining of Mac-2bp revealed that hepatocytes strongly expressed Mac-2bp in patients with NAFLD 16. It is necessary to investigate whether HSCs are the only cells that produce M2BPGi in the blood 18. WFA is known to recognize the structure LacdiNAc 19, but M2BPGi actually only captures Mac-2bp with this sugar chain structure, implying that complex formation in blood is important for lectin recognition.