Tadasu Urashima
Obihiro University of Agriculture & Veterinary Medicine, Obihiro, Japan. Ph.D., Agriculture.
After graduation from Tohoku University (Doctor of Agriculture) in 1986, he started his professional career by studying milk oligosaccharides at Obihiro University. In 1991, he studied glycosyltransferase activity in lactating mammary glands of the tammar wallaby (marsupial) under Dr. Michael Messer in the Department of Biochemistry, the University of Sydney. Then he completed a comprehensive study of milk oligosaccharides of monotremes, marsupials, and several species of eutherians with Dr. Messer. He is interested in how the present milk components have been acquired during the evolution of mammals, especially how the acquisition of α-lactalbumin, a milk protein, has resulted in the appearance of milk oligosaccharides and lactose, and caused their biological significance to change during evolution. Since 2003, he has been a full professor at Obihiro University. At present he is president of the Japanese Society of Dairy Science and a board member of the Japanese Society of Carbohydrate Research (JSCR) and Japanese Consortium for Glycoscience and Glycotechnology (JCGG).
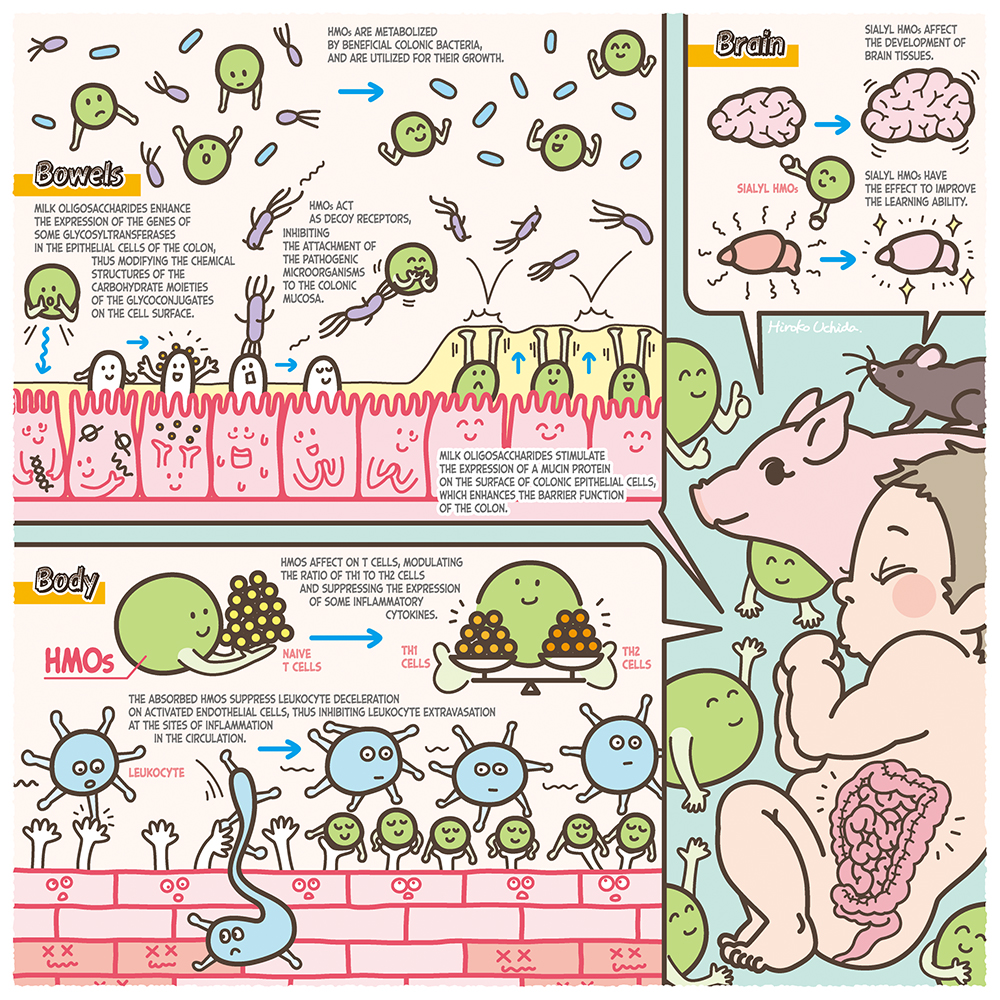
Human milk contains 7% of carbohydrate, 80% of which consists of lactose (Galβ1-4Glc), while 20% consists of oligosaccharides. The concentrations of milk oligosaccharides are 12 ~ 13 g/L in mature milk and 22 ~ 24 g/L in colostrum1, these are the third largest solid component after lactose and lipid. Their concentrations are surprisingly high. Most of the human milk oligosaccharides (HMOs), with few exceptions, contain a lactose unit at their reducing ends, to which monosaccharides residues including N-acetylglucosamine (GlcNAc), galactose (Gal), fucose (Fuc), and N-acetylneuraminic acid (Neu5Ac) are attached. To date about 250 HMOs have been separated, of which about 170 structures have been characterized1. When breast-fed infants consume their mothers’ milk, the lactose is hydrolyzed to Glc and Gal by small intestinal lactase, to be absorbed, whereas most HMOs remain intact within the small intestine and thus reach the colon, where they have significant physiological functions other than nutritional effects2,3. Based on the experimental evidence, the following functions have been proposed: stimulation of the growth of beneficial colonic bacteria such as bifidobacterium, anti-infection against pathogenic bacteria and viruses, immune modulation including anti-inflammation, prevention of necrotizing enterocolitis and reinforcement of the colonic barrier function, and activation of brain-nerve functions3. In this series of Glycoforum, some of the exciting discoveries will be described, focusing on the metabolic pathways of HMOs by bifidobacteria, the functions of HMOs relating to the cross talk between colonic bacteria and host epithelial cells, and the industrial production of HMOs. In this series the history of studies in this field, with future aspects, will be dynamically expanded by first-class researchers. In the beginning of this series, international studies clarifying the biological functions of HMOs will be introduced.
Previously, most studies of anti-infection against pathogens by HMOs had been performed using in vitro experiments with co-incubation of the epithelial cells and bacteria or virus in the presence/absence of HMOs. Inhibitions of the infection by HMOs was evaluated from the decrease in the counts of the pathogens adhering to the cells compared with the counts in absence of HMOs. For example, Hester et al. counted the levels of rotavirus of pig or human origin which adhered to MA-104 cells, derived from Macacus rhesus monkey kidney, after the medium was cultured with an 1 ~ 10 mg/mL HMOs mixture. They found that when a pig origin virus was utilized the count level decreased compared with that of the control experiment without HMOs, but there was no decrease in the experiments using a human origin virus4. When a different human origin rotavirus strain was cultured with MA-104 cells in the presence of 2’-FL, 3’-SL or 6’-SL, the count levels of the virus adhering to the cells decreased in the presence of 5.0 mg/mL of 6’-SL compared with those in the control5. However, the adhered virus levels increased in other experiments using another human origin rotavirus strain6, suggesting that positive evidence for anti-infection by HMOs was not always obtained and was not straightforward.
Some studies suggest anti-infection by enhancement of a vaccine effect by feeding of HMOs but not by an anti-adhesive mechanism. Xiao et al. fed a diet containing 0.25 ~ 5% of 2’-FL, the most predominant HMO, to 6 female mice aged 6 weeks and then loaded the inactivated influenza virus into the right ear, with saline water into the left ear two weeks later, and further vaccinated with the inactivated virus nine days later7. They evaluated the inflammatory reaction by measurement of the thickness of both ears before re-vaccination and 24 days after. The results showed an increase in the inflammatory reaction of the right ear and also of concentrations of the virus specific IgG1 and IgG2 levels in the serum. In another study, piglets were fed with milk replacer containing 4 g/L HMOs and were then orally administrated with the rotavirus strain. A few days after the administration, the counts of the immunocytes were measured and compared with those of the control animals fed with no HMOs; the results showed that the counts of interferon-γ producing cells were twice as high in the peripheral blood of the HMOs fed animals compared with those of the control animals8. This suggests the possibility of an anti-infection mechanism of enhancement of innate immunity by the feeding of HMOs.
Anti-infection by inhibition of the attachment to epithelial cells has also been observed for pathogenic bacteria. After HeLa cells, Hep-2 cells or T84 cells were infected with precultured entero pathogenic Escherichia coli strain and incubated with 10 mg/mL of HMOs, the bacterial counts, which adhered to the cells, were measured and compared with those of the controls with no preincubation with HMOs. The data showed that the adhered bacterial counts to the cells were significantly decreased after incubation with HMOs compared with those with no HMOs9.
Observations indicating that HMOs directly inhibited the growth of pathogenic bacteria have been also reported. After group B Streptocossus (GBS), which causes the meningitis, was cultured in the presence of 0.25 ~ 2 mg/mL of neutral HMOs, the bacterial growth was significantly inhibited in the medium containing HMOs compared with no HMOs10.
The above-mentioned anti-infection effects of HMOs were observed in in vitro and in vivo experiments, but it is necessary to obtain data from clinical experiments with people infected with the pathogens. Since industrial scale productions of a few HMOs, such as 2’-FL, 3’-SL and LNnT has begun, such clinical studies will soon be possible.
Although most HMOs are not hydrolysed in the small intestine, there is evidence that small amounts of HMOs are absorbed, thus entering the circulation where they act as possible immune modulators, including anti inflammation. For example, the following data from in vivo experiments have been reported. Catillo-Coutade et al. orally fed 5 mg/ml of 2’-FL or 6’-SL to ovalbumin sensitized mice for some period and observed the incidence of diarrhea and malnutrition, and measured inflammatory cytokine production in spleen cells and mast cells. The results were compared with those obtained with control animals fed with no HMOs11. They observed that the feeding of HMOs reduced the frequency of diarrhea and malnutrition and also reduced the counts of mast cells and the production levels of inflammatory cytokines such as tumor necrosis factor (TNF).
Reduced incidence of food allergy produced by HMOs has been suggested by data from in vitro experiments. Zehra et al. cultured T84 or HT29 cells with antigen-antibody complex in the presence/absence of 10 mg/mL of 2’-FL or 6’-SL and determined the secretion levels of inflammatory cytokine IL-8 or CCL20. They found that the IL-8 level was decreased in the media containing T84 or HT29 cells by addition of 2’-FL compared with that with no 2’-FL, and the levels of both IL-8 and CCL20 were decreased in the media containing T84 cells by the addition of 2’-FL when compared with the control12.
There are studies suggesting potential immune modulation in the circulation through the interaction between leukocytes and absorbed HMOs. Zhang et al. cultured the media of RAW264.7 cells originating from mouse ascites macrophagea, in the presence/absence of 0.1 ~ 1.0 g/mL HMOs, and determined the levels of inflammatory mediators or cytokines. They found increases in the expressed levels of mRNA of IL-1β, IL-2, IL-6, IL-10 and TNF-α and also in the productions of inner nuclear NfkB proteins, p38 kinase, extracellular signal related kinase and C-Jun terminal kinase13. These data suggest activation of NfkB and MAPKs signaling pathways triggered by HMOs.
Although it is known that the frequency of NEC in breast-fed infants is lower than that in bottle fed infants, it was also shown that feeding of HMOs had the effect of prevention and reducing the symptomatology of NEC. Jantsher-Krenn et al. produced NEC in newborn rats by injection of proinflammatory agents into the colons of pregnant rats14. The newborns were grouped into a DAM group, that was bred by their mothers, an HMOs group, fed with milk replacer containing 10 mg/mL of HMOs, and a control group, fed with milk replacer containing no HMOs; the colons were collected after 96 hr. When inflammation of the colons of each group was measured, the authors found that the inflammation in the HMOs group was less than that in the control group, and was similar to that in the DAM group. When the authors compared the oligosaccharides, they found that DSLNT, which contains two moles of sialic acid, was the most effective in reducing inflammation.
From the above observations it was hypothesized that the mechanism for protection against NEC development by feeding HMOs could be enhancement of colonic barrier function. Wu et al. produced NEC in neonatal mice by exposure to hypoxia and feeding of lipopolysaccharide (LPS), and fed the mice with 20 mg/mL of HMOs, followed by collection of the colons to score inflammation15. They found that the score values for inflammation were improved in the HMOs fed mice when compared with the control animals that had not been fed with HMOs. Microscopic observation of the colonic tissue showed that the most serious damage was found in Muc2 producing colon epithelial cells of the control animals compared with the HMOs fed animals, and that the cell numbers were higher in HMOs fed mice than in the controls. Muc2 is the secretory mucin, mainly produced by goblet cells located in the large as well as in the small intestine, which are considered to be important for maintenance of the barrier function.
It has been suggested that the sialic acid absorbed after feeding sialyl HMOs can be utilized to synthesize brain sialyl glycoconjugates. Jacobi et al. fed milk replacer containing 2 ~ 4 g/L of 3’-SL or 6’-SL to piglets for 21 days; after collection of the piglet brain tissue they determined the concentrations of total sialic acid as well as of gangliosides in the cerebral cortex, cerebellum, corpus callosum and hippocampus16. They found that the concentrations of total sialic acid and gangliosides in the cerebellum were higher in a dose dependent manner in the 3’-SL fed animals than in the controls that had been fed milk replacer without SL, while the total sialic acid concentration in the corpus callosum was increased in animals fed with milk replacer containing 2 g/L of 3’-SL or 6’-SL.
There are studies exploring whether brain tissues would expand after the feeding of sialyl HMOs. Mudd et al. determined the concentrations of sialic acid in the brain tissues of piglets that were fed with milk replacer containing 0 ~ 760 mg/L of sialyllactose (SL), and also examined the brain tissues with a magnetic resonance imaging (MRI) instrument to determine any correlation between SL feedings and brain tissue development, specifically of expansion of the hippocampus in axial and radial directions17. The results showed that expansion was found in this tissue when medium level concentrations of SL were fed to the animals, though not in a dose – dependent manner, while the ratio of concentration of free sialic acid to sialic acid bound in glycoconjugates in the hippocampus was higher in SL fed animals than in the control animals.
Other studies suggested that feeding of HMOs affects the behavior of experimental animals. Tarr et al. bred three male mice in separate cages in which they were fed diets for one week in the presence/absence of 5% of 3’-SL or 6’-SL18. The mice were then exposed to an aggressive mouse for two hours per a day, for one week in each cage, after which anxiety-like behavior was observed. It was found that this behavior had improved in the SL fed mice when compared with the control animals that had not been fed with 3’-SL or 6’-SL. These results suggested that sialic acid, originating from sialyl HMOs, was absorbed and utilized for the synthesis of brain gangliosides, and that this could be related to the development of brain tissue, affecting the animals’ behavior.
There are studies suggesting that the exposure of colonic epithelial cells to HMOs affects the expression of the glycogenes that modify the carbohydrate structures of cell surface glycoconjugates. When Caco-2 cells were exposed to 3’-SL, the expression level of ST3Gal1,2,4 α2,3-sialyltransferase was decreased, and it was suggested that the glycan structures on the cell surface were altered19. This could have reduced the expression levels of α2,3 and/or 2,6 linked sialyl glycoconjugates, and this might have the effect of decreasing the attachment of entero pathogenic E. coli to the colonic epithelial cells.
After HT-29 cells were exposed to sialyl milk oligosaccharides separated from bovine colostrum (BCO), it was observed that modification occurred in the glycoconjugates on the cell surface and that the attachment of normal resident microbiota was stimulated. Morrin et al. cultured HT-29 cells in a medium in the presence/absence of 0.5 ~ 6 mg/mL of BCO or 3’-SL, after which strains of Bifidobacterium longum subsp. infantis, B. longum subsp. longum, B. breve, B. adolesentis, or Lactobacillus rhamnosus were added to be further cultured, followed by collection of the bacteria attached to the cells after washing20. After the bacteria were cultured on an agar medium to count the colony numbers, it was found that the bacterial numbers adhering to the cells increased 22 times for B. longum subsp infantis, 52 times for B. longum, and 24 times for B. breve after exposure to BCO, compared to the controls which had not been exposed. It was shown that in BCO exposed cells, the expression levels of mRNAs of five glycosyltransferases (STSGAL5, CHST4, GNE, B4GALNT3 and B5GNT5) were increased, suggesting that this was related to enhancement of attachment levels of the above Bifidobacteria to the epithelial cells.
Even though the recently reported biological functions of HMOs and BCO were mentioned early in the “Milk oligosaccharides series”, there are only a few examples of studies concerning their biological significances. Furthermore, the molecular basis, such as the interaction between oligosaccharides and some lectins, has not yet been clarified for many biological functions. I emphasize that exploration of their functions by studies of their molecular basis must be an important future task.
In this mini review I have introduced recent functional studies performed in overseas countries. In the following mini reviews in this series, domestic front line researchers will introduce the metabolic pathways of HMOs by bifidobacteria, cross talk between the host and the colonic bacteria, and industrial utilization of HMOs, among other topics.
The illustration of the functions of HMOs will be added as a figure later.