
Tadasu Urashima
After graduation from Tohoku University (Doctor of Agriculture) in 1986, he started his professional career by studying milk oligosaccharides at Obihiro University. In 1991, he studied glycosyltransferase activity in lactating mammary glands of the tammar wallaby (marsupial) under Dr. Michael Messer in the Department of Biochemistry, the University of Sydney. Then he completed a comprehensive study of milk oligosaccharides of monotremes, marsupials, and several species of eutherians with Dr. Messer. He is interested in how the present milk components have been acquired during the evolution of mammals, especially how the acquisition of α-lactalbumin, a milk protein, has resulted in the appearance of milk oligosaccharides and lactose, and caused their biological significance to change during evolution. In 2003 ~ 2022, he had been a full professor at Obihiro University, and is an honorary professor today. At present he is a secretary of Japanese Consortium for Glycoscience and Glycotechnology (JCGG).

Takashi Ishida
Sales and Marketing Department, Kyowa Hakko Bio Co., Ltd.
2009, Master of Science, Graduate School of Life Science and Technology, Tokyo Institute of Technology.
2009, Joined Kyowa Hakko Bio, Research Scientist
2017–2018, Corporate sales for raw materials and OEM business
2018–2024, Business development for human milk oligosaccharides, concurrently working at Kirin Holdings Company Limited
2025–present, Manager, Sales and Marketing Department
His interests focus on the development and application of functional food ingredients. He is committed to advancing raw material development and bridging academic research with practical business solutions.

Takane Katayama
Professor, Graduate School of Biostudies, Kyoto University
Takane Katayama is a Professor at Kyoto University. He received his Ph.D in the field of applied molecular microbiology under the supervision of Prof. Hidehiko Kumagai. After spending three years as a postdoc in the same lab, he moved to the lab led by Prof. Kenji Yamamoto and was appointed an assistant professor. In Prof. Yamamoto’s lab, he isolated the genes for 1,2-α-L-fucosidase and endo-α-N-acetylgalactosamindase from bifidobacteria, both of which are enzymes acting on human-derived glycans. These findings prompted him to consider host glycans-mediated symbiosis between gut microbes and humans. In the last decade, he has focused on functional analysis of bifidobacterial genes and enzymes involved in degradation of human milk oligosaccharides. His research has significantly contributed to revealing how bifidobacteria-rich microbiota is formed in the gut of breast-fed infants.
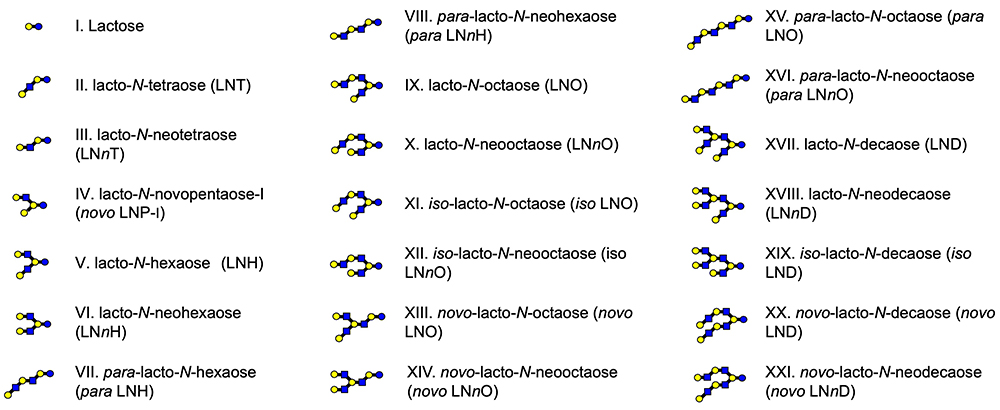
Human mature milk contains around 11.3 g/L of human milk oligosaccharides (HMOs) as an average concentration, which is the third largest solid component after lactose and lipid1. Around 200 HMO structures have been characterized to date, and they are classified into 21 series based on core structures, none of which contain fucose (Fuc) and N-acetylneuraminic acid (Neu5Ac) (Fig 1)1. Representative HMO structures are shown in Fig 2. Other HMOs exist only in trace amounts in human milk. When breast-fed infants drink their mothers’ milk, a major part of the HMOs is not absorbed in the small intestine to reach the colon. HMOs perform the following biological functions: (1) they stimulate the growth of the beneficial colonic bacteria including bifidobacteria, (2) they inhibit infection by pathogenic microorganisms, (3) they affect immune modulation, (4) they inhibit the development of necrotizing enterocolitis (NEC) by strengthening colonic barrier function, and (5) they stimulate nerve and brain function, etc.
At present, only a few HMOs have been manufactured on an industrial scale and used as additives to infant formula in several overseas countries, and the number of cohort studies showing correlation between the health status of breast-fed infants and the profile of HMOs in their mothers’ milk, as well as in vivo or in vitro studies, demonstrating biological functions is rapidly increasing. We summarize the history of HMOs studies in Japan in this mini review, and address what the future may hold for HMOs studies as well as steps toward commercializing HMOs in Japan.
The first HMO study explored growth stimulating factors of bifidobacteria, the beneficial bacteria found in infant colon. In 1886, Escherich demonstrated that the colonic bacterial condition is related to survival rate in breast-fed infants, and that carbohydrate in the breast milk might stimulate the growth of the bacteria2. In 1888, based on his study of human and bovine milk carbohydrates, Eschbach reported that the type of carbohydrate in human milk was different from that in bovine milk3. At that time, mortality risk was seven times higher in infants, who were bottle-fed manufactured formula than in infants who were breast-fed, because breast milk reduces the development of diarrhea-causing infections in infants3. In 1900, Tisser compared the colonic microflora of breast-fed infants to that of bottle-fed infants4, and in 1900, Moro had found that Lactobacillus bifidus, which is considered to be a bifidobacterial species today, was more prominent in the colon of breast-fed infants than in that of bottle-fed infants5. In 1926, Schönfeld found that human breast milk contained a component, that promoted the growth of bifidobacteria in the colon6. In the 1930s, Polonowski and Lespagnol found human milk contained a methanol insoluble carbohydrate, which they named “gynolactose”7,8. With Montreuil, they studied gynolactose using two dimensional paper chromatography and found several components, not a single homogeneous one9.
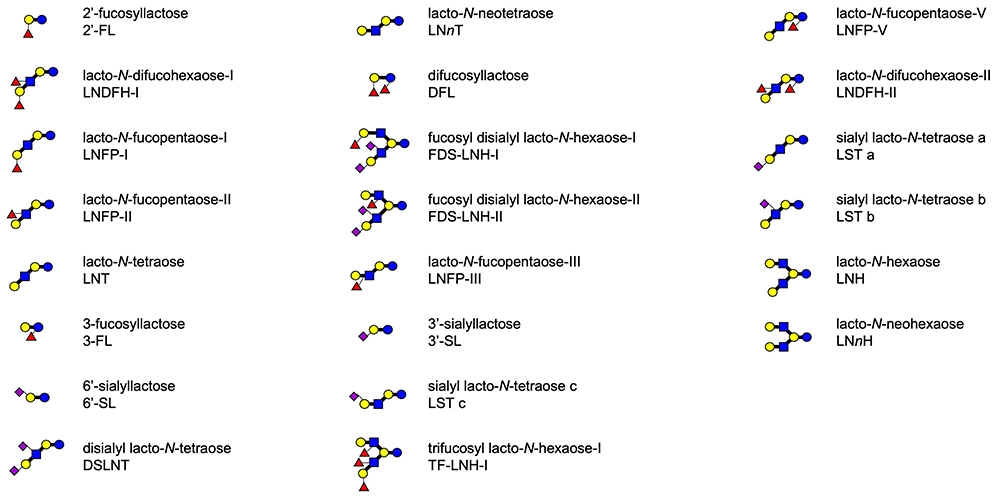
In the 1950s, Kuhn and György began purifying and structurally characterizing the components in gynolactose to identify the bifidobacterium growth stimulation factor in human breast milk10. By 1965, fourteen oligosaccharides including lacto-N-tetraose (Galβ1-3GlcNAcβ1-3Galβ1-4Glc, LNT), lacto-N-neotetraose (Galβ1-4GlcNAcβ1-3Galβ1-4Glc, LNnT) and their fucosyl or sialyl derivatives as well as fucosyl or sialyl lactoses had been characterized mainly by Kuhn11.
Summaries of the studies on HMOs in Japan are shown in Fig 3 and Fig 4. The first HMOs study was performed by Akira Kobata of the Medical School of Kobe University and the Institute of Medical Science of Tokyo University. When he had worked in Takeda Pharmaceutical Company Ltd., he investigated the nucleotides in human breast milk, almost none of which were found in bovine milk, and discovered unique sugar nucleotides, uridine diphosphates (UDP) with covalently bound di- or tri-saccharides. These sugar nucleotides were identified as Galβ1-4GlcNAcα1-UDP and Fucα1-2Galβ1-4GlcNAcα1-UDP12,13. Oligosaccharide nucleotides have been detected in the milk or colostrum of a few species. Roseman’s group identified Siaα2-3(6)Galβ1-4GlcNAcα1-UDP and Siaα2-3(6)Galβ1-6GlcNAcα1-UDP in caprine colostrum14, while Urashima’s group found Galβ1-4GlcNAcα1-UDP in reindeer milk15, and Neu5Gcα2-3Galβ1-4GlcNAcα1-UDP and Neu5Gcα2-6Galβ1-4GlcNAcα1-UDP in ovine colostrum16. However, the physiological significance of such molecules in milk or colostrum remains unclear, because they are not always detected in milk and colostrum.
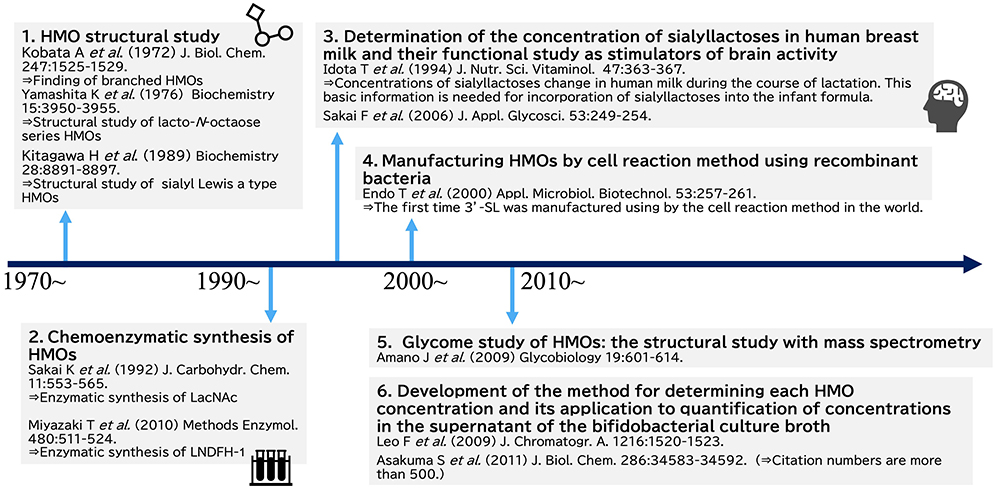
Based on the finding of Galβ1-4GlcNAcα1-UDP and Fucα1-2Galβ1-4GlcNAcα1-UDP in human milk, Kobata hypothesized that glycosyltransferase in milk catalyzes di- or trisaccharide transfer from these sugar nucleotides to an acceptor. In order to find such glycosyltransferase activity, he studied in the laboratory of Paul György at the University of Penssylvania. Unfortunately, this enzymatic activity had never been detected in breast milk11,17 and oligosaccharyl transferase activity had never been detected in nature up to this time. Then he changed the focus of his research at this University to the biosynthetic pathways of human blood group A or B antigens. The reaction had been studied using UDP-GalNAc as the donor and 2’-fucosyllactose(Fucα1-2Galβ1-4Glc, 2’-FL)or lacto-N-fucopentaose- I (Fucα1-2Galβ1-3GlcNAcβ1-3Galβ1-4Glc, LNFP- I)as the acceptor, with the breast milk of blood A or AB group donors serving as the enzymatic resource to explorer the production of the A-tetrasaccharide [GalNAcα1-3(Fucα1-2)Galβ1-4Glc] or A-hexasaccharide [GalNAcα1-3(Fucα1-2)Galβ1-3GlcNAcβ1-3Galβ1-4Glc]11,17. Another reaction had been studied using UDP-Gal as the donor and 2’-FL or LNFP- I as the acceptor, with the breast milk of blood B or AB group donors used as the enzymatic resource to explorer the production of B-tetrasaccharide [Galα1-3(Fucα1-2)Galβ1-4Glc] or B-hexasaccharide [Galα1-3(Fucα1-2)Galβ1-3GlcNAcβ1-3Galβ1-4Glc]18,19. From these data the biosynthetic pathways of blood group A and B antigens were elucidated.
After returning to Japan, he continued to purify and characterize HMOs from breast milk in order to use them as substrates in studies of some glycosyltransferases or glycosidases. In 1972, he identified lacto-N-hexaose [Galβ1-3GlcNAcβ1-3(Galβ1-4GlcNAcβ1-6)Galβ1-4Glc, LNH] and lacto-N-neohexaose [Galβ1-4GlcNAcβ1-3(Galβ1-4GlcNAcβ1-6)Galβ1-4Glc, LNnH], the first branch type HMOs to be recognized20,21, and then with Katsuko Yamashita, identified their fucosyl and sialyl derivatives22,23 as well as several other HMOs whose core structures were para LNH (linear structure, see Fig 1)24, para LNnH (linear structure, see Fig 1)24, lacto-N-octaose (LNO) (branch structure, see Fig 1)25,26, or lacto-N-neooctaose (LNnO) (branch structure, see Fig 1)25,26. Before Kobata et al., only the linear tri- to hexa-saccharide HMOs with lactose, LNT, or LNnT core units had been characterized. It is no exaggeration that the results of his studies became the starting point for further studies focused on the diversity of HMOs with a lot of complicated structures.
Purified and characterized HMOs from human breast milk have been utilized to study the substrate specificity of some glycosyltransferases and glycosidases and to analyze the sugar epitopes of lectins including galectins. Table 1 shows previous HMOs that were commercially available from a Swedish company in 1980s, and highlights the structures characterized by Kobata and Yamashita. Today the operation of businesses that commercialize HMOs separated from human milk seems to be shrinking because of ethical issues. However, the use of Kobata and Yamashita’s HMOs as carbohydrate standards must have undoubtedly made a large contribution to the advancement of glycobiology.
In the early part of the 20th century as well as 19th century, bottle-fed infants had very low abundance of bifidobacteria in colonic microflora, and it was thought that the cause was the difference in concentration of oligosaccharides in milk between humans and cows. To enhance the abundance of bifidobacteria in the microflora of bottle-fed infants, galacto oligosaccharides, which are manufactured from lactose by the transgalactosylation reaction using β-galactosidase from lactic acid bacteria, have been developed as infant formula additives. This technology was mainly developed by Japanese companies. The products contain 6’-galactosyllactose (Galβ1-6Galβ1-4Glc, 6’-GL). As 6’-GL was identified in human breast milk by Kobata and Yomashita27, its existence in human breast milk must have spurred the production of galacto oligosaccharides for use as additives in infant formula.
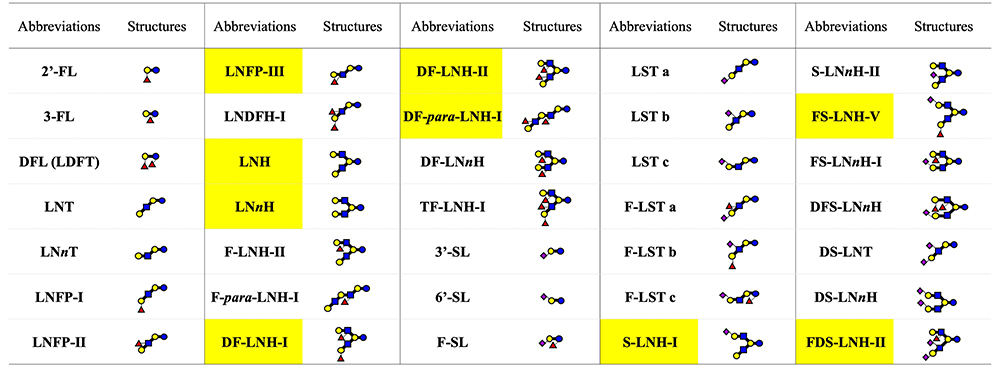
Since the study by Kobata and Yamashita, the chemical structures of HMOs continued to be characterized, and to date, around 200 structures classified into 21 series based on core structures have been identified1. Among the notable studies carried out in Japan is the series on structural characterization conducted in 1988 - 1993 by Hiroyuki Kitagawa (Kobe Pharmaceutical University at present) at the laboratory of Ikuo Yamashina in Kyoto University11. To determine the structure of the sugar epitope of a monoclonal antibody MSW113 to one antigen on some cancer cells, Kitagawa et al. characterized the structures of HMOs that had been separated from the carbohydrate fraction of breast milk by affinity column chromatography using MSW113. As the result, a series of HMOs structures, whose core units were LNT, LNH, LNO as well as iso LNO (see Fig 1), were identified to have a sialyl Lewis a unit (Neu5Acα2-3Galβ1-3[Fucα1-4]GlcNAc) at the non-reducing ends; many of them had novel structures11,28-30.
In 2009, Junko Amano (Noguchi Institute) et al. identified 22 HMOs structures with core units were lacto-N-decaose (LND, see Fig 1) or lacto-N-neodecaose (LNnD, see Fig 1) isolated from the breast milk of A, Leb+ group blood donors31. In this study, they characterized the structures by using negative-ion matrix-assisted laser desorption/ionization-quadrupole ion trap-time of flight mass spectrometry (MALDI-QIT-TOFMS) after fractionation of the derivatives with pyrene reagent by the reverse phase - high performance liquid chromatography (HPLC) as the mobile phase. Currently, analysis with tandem mass spectrometry has become mainstream for the structural characterization of HMOs, as it is convenient for analyzing a very small amount of breast milk. The study by Amano et al. can be said to be a pioneering.
A few HMOs including 2’-FL, 3’-sialyllactose (Neu5Acα2-3Galβ1-4Glc, 3’-SL), 6’-sialyllactose (Neu5Acα2-6Galβ1-4Glc, 6’-SL), 3-fucosyllactose [Galβ1-4(Fucα1-3)Glc, 3-FL], LNT, and LNnT are presently manufactured on an industrial scale by the fermentation method using recombinant bacteria and incorporated into commercial infant formula overseas. However, the practice of adding HMOs, manufactured by recombinant bacteria to commercial foods, has been banned by the consumer affairs agency in Japan. It is hoped that the use of such products in commercial foods will be permitted after their safety is confirmed in the near future, as it has been proven that HMOs have many beneficial biological functions. On the other hand, the sialyllactoses, which are present in human milk, were added to the infant formula for the first time in the world by a Japanese company to enhance brain function and nerve activity.
Because the brain contains large amounts of gangliosides and polysialyl glycoproteins, it was hypothesized that the sialyl oligosaccharides in mother’s milk would be utilized as the materials to biosynthesize the sialyl components in the brains of breast-fed infants. In fact, Jacobi et al. (2016) demonstrated that the ganglioside concentration in the cerebellum as well as the concentration of sialic acid in the corpus callosum of piglets was enhanced after the feeding of 3’-SL or 6’-SL for 21 days32. Mudd et al. (2017) used magnetic resonance imaging to find that the volume of corpus callosum and left hippocampus were expanded in the piglets fed sialyllactoses, although this expansion rate was not dose dependent33.
As the sialic acid concentration in human breast milk differs from that in bovine mature milk, which is used as a material for manufacturing infant formula, Snow Brand Milk Products Co., Ltd. (MEGMILK SNOW BRAND Co., Ltd. at present) has separated sialyllactoses, which are the mixture of 3’-SL and 6’-SL, from cheese whey, and has been adding them to the company’s infant formula since the 1990s34. To establish a scientific basis for adding sialyllactoses to the company’s infant formula, Tadashi Idota (Snow Brand Milk Products Co., Ltd.) et al. (1994) determined the concentrations of 3’-SL and 6’-SL in 2279 milk samples collected from 2434 Japanese women at 3 - 482 days post-partum35. In addition to this study, the study of Fumihiko Sakai (Snow Brand Milk Products Co., Ltd.) et al. (2006) showed that rats fed sialyllactoses tended to have higher levels of gangliosides such as GM3 (Neu5Acα2-3Galβ1-4Glc-Cer) and greater swimming learning ability in the Morris water maze test36.
Trials to use sialyllactoses, separated from cheese whey, have been conducted to see whether they can improve the nutritional status of malnourished infants in the developing countries. Charbonneau et al.(2016) and Cowardin et al. (2019) in the group of Jeffrey Gordon took colonic bacteria isolated from the feces of malnourished breast-fed infants, to germ-free mice and piglets. They fed the animals a sialyllactoses-containing diet or control diet and measured body weight and bone density37,38. They observed increased body weight and bone density in the sialyllactoses-fed animals than in the control diet-fed animals. This trial might be related to the pioneer work by Snow Brand Milk Products Co. Ltd as above.
Studies of the chemoenzymatic synthesis of HMOs using glycosidases or glycosyltransferases are ongoing, even though glycosidases or glycosyltransferases are not practical for the manufacture of HMOs on an industrial scale. Tatsuo Miyazaki in the group of Katsumi Ajisaka (Meiji Health Science Institute, Niigata University of Pharmacy and Medical and Life Sciences) synthesized lacto-N-difucohexaose- I (Fucα1-2Galβ1-3[Fucα1-4]GlcNAcβ1-3Galβ1-4Glc, LNDFH- I) by the enzymatic reaction using N-acetylglucosaminyltransferase and fucosyltransferase as well as the reverse reaction with β-galactosidase39.
A few HMOs including 2’-FL, 3-FL, 3’-SL, 6’-SL, LNnT and LNT are currently being manufactured using the recombinant bacteria on an industrial scale, and infant formula containing 2’-FL and LNnT is commercially available in 50 overseas countries. As products by recombinant bacteria other than highly refined products have not been permitted yet to be food additives by the consumer affairs agency in Japan, the utilization of human-identical milk oligosaccharides (HiMOs), manufactured by this method, has been delayed in Japan. However, the production method of HMOs using the bacterial cell reaction was first developed by Japanese scientists.
In bacterial cell reaction, sugar nucleotides are produced by the catalytic actions of several gene-expressed enzymes within cells, and the egress of the products from the inside to the outside of the cell is enhanced by addition of the surfactant or organic solvent. When recombinant bacteria expressing some glycosyltransferases are cultured with the obtained sugar nucleotides and acceptor sugars, the target HMOs are produced in the bacterial cells. In 2000, Tetsuo Endo and Satoshi Koizumi (Kyowa Hakko Bio Co. Ltd.) showed that cytidine monophosphate (CMP)-Neu5Ac was produced and accumulated with orotic acid and sialic acid in recombinant Escherichia coli and Corynebacterium ammoniagenes, expressing both of CMP sialic acid synthetic and cytidine triphosphate (CTP) synthetic enzymes. Then 3’-SL was produced in the recombinant E. coli, in which α2,3 sialyltransferase from Neisseria gonorrhoeae was gene-expressed in the biosynthetic system of CMP-Neu5Ac40. This process should be regarded worldwide as a pioneering one in the industrial productions of HMOs, as it succeeded more than 10 years before overseas companies including DSM Co. Ltd. and Novonesis Co. Ltd. attempted to do in the similar concept.
Kyowa Hakko Bio Co. Ltd. (at that time) developed the production systems for the production of 2’-FL, 3’-SL, and 6’-SL, despite the ban on their commercial utilization in Japan. The system uses a fermentation method involving synthesis of sugar nucleotides, and expression of glycosyltransferases in same bacterial cells; this is more sophisticated than the bacterial cell reaction method. 3’-SL and 6’-SL can be produced in recombinant E. coli, in which Neu5Ac is produced from glucose as substrate, by introducing genes encoding CMP sialic acid synthetic enzyme and N-acetylneuraminyltransferase, while 2’-FL can be produced in the recombinant E. coli, in which the biosynthetic system of guanidine diphosphate (GDP)-Fuc is improved, and the gene of α2-fucosyltransferase is introduced41.
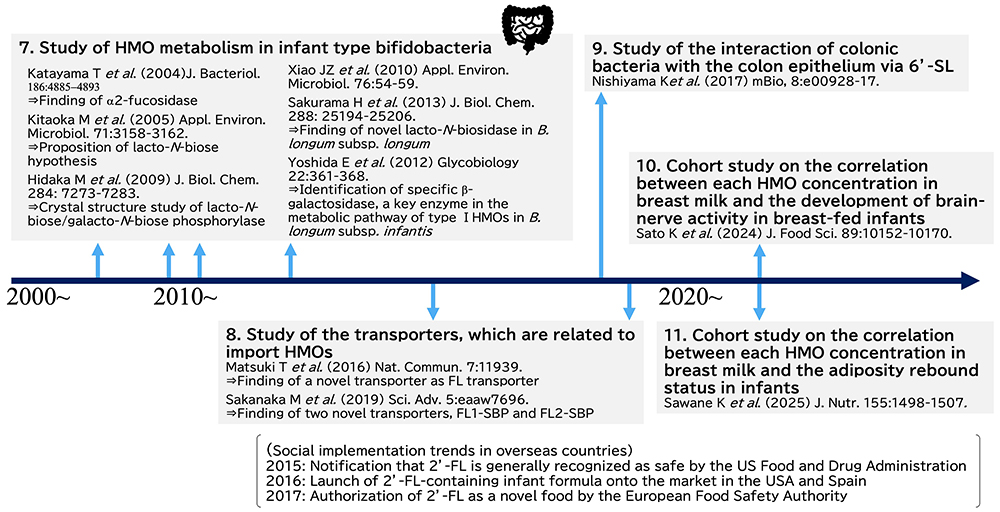
At the beginning of the study of the HMO metabolic pathways in bifidobacteria, four species of infant type bifidobacteria, including Bifidobacterium bifidum, B. longum subsp. infantis, B. longum subsp. longum and B. breve, were cultured in the semi-synthesized broth containing HMOs as the only carbon source, and then the concentration of each HMO remaining in the supernatant were determined at several time points during the incubation. For this study, a method was needed to determine precisely the concentration of each HMO. Although, to date, a huge number of the papers have been published on the concentration of each HMO in human milk, it is rather recent, i.e., in the 2000s, that a method for determining this concentration, such as by high performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD), has been developed. In previous studies, Urashima’s group quantified the concentration of each HMO in breast milk using normal phase HPLC after the derivatization of HMO with pyridyl amine (PA) or 2-amino benzoic acid (2-AA)42,43, and then applied the method with 2-AA to determine the concentration of each HMO in the supernatant of broth cultured bifidobacteria, which had been incubated with breast milk HMOs44. Sadaki Asakuma (Hokkaido Agricultural Research center) et al. (2011) used this quantification method to examine how each of the four infant type Bifidobacterium species utilizes HMOs during in vitro cultivation, and found the different characteristic pattern of HMO consumption by B. bifidum and B. longum subsp. infantis44. This pioneer study for consumption of HMOs by bifidobacteria has been cited in over 500 papers up to date.
Even though it had been traditionally known that HMOs stimulate the growth of bifidobacteria in the colon of breast-fed infants, the study clarifying the HMO metabolic pathways of these species started just in the 2000s. Japanese scientists contributed greatly to the clarification of these pathways in four species of the infant type bifidobacteria. Table 2 shows the representative studies on this topic in Japan. In B. bifidum, HMOs are degraded by extracellular enzymes into mono- and di-saccharides. Namely sialic acid and Fuc are released from sialyl or fucosyl HMOs by the action of extracellular sialidase and fucosidase, respectively, during their metabolism, and then lacto-N-biose-I (Galβ1-3GlcNAc, LNB) is released from type 1 HMOs such as LNT by the action of lacto-N-biosidase. These extracellular enzymes were purified and cloned by members of the Takane Katayama and Hisashi Ashida (currently Kinki University) research group at Kyoto University45-47. LNB is transported into the cell interior by the action of a specific transporter, and then phosphorolyzed to Gal-1-P and GlcNAc by the action of LNB/galacto-N-biose (GNB) phosphorylase48. GlcNAc is converted to UDP-GlcNAc by the action of N-acetylhexosamine 1-kinase and UDP-glucose hexose-1-phosphate uridyltransfarase49. This intracellular metabolic pathway was proven by the group of Motomitsu Kitaoka of the National Food Research Institute (currently Niigata University).The intracellular metabolic pathway of LNB was studied with the strain of B. longum subsp. longum at first. Then it was shown that four infant type bifidobacteria commonly possess this pathway. As Kitaoka and Nishimoto developed the method of LNB production on an industrial scale50, LNB should be potential prebiotic material to be added into the functional foods. On the other hand, B. bifidum does not have systems for metabolizing Neu5Ac and Fuc, which are released from HMOs by the actions of extracellular glycosidases. In the study by Asakuma et al., it was shown that Fuc as well as Gal remains unconsumed in the supernatant of broth cultures of B. bifidum, when incubated in the broth supplemented with neutral HMOs as the only carbon source44. As these monosaccharides can be utilized by B. breve and B. longum subsp. longum, whose HMO utilization capability is limited, it is hypothesized that B. bifidum cross feeds these bifidobacteria in the breast-fed infant colon. B. breve can use Neu5Ac for growth, but not 6’-SL. Keita Nishiyama (currently Tohoku University) and Takao Mukai of Kitasato University showed that B. breve can grow on 6’-SL upon co-incubation with B. bifidum, while it does not grow on the sugar when co-incubated with sialidase-knockout B. bifidum, indicating the potential cross feeding of sialic acid from one species to the other51. Aina Gotoh of Katayama’s group found that when feces collected from infants born via Cesarean section, weaned infants, or adults were incubated with B. bifidum strain in the culture medium containing HMOs, the number of Bifidobacterium species other than B. bifidum increased in the medium; this suggests potential cross feeding between B. bifidum and other bifidobacteria52.
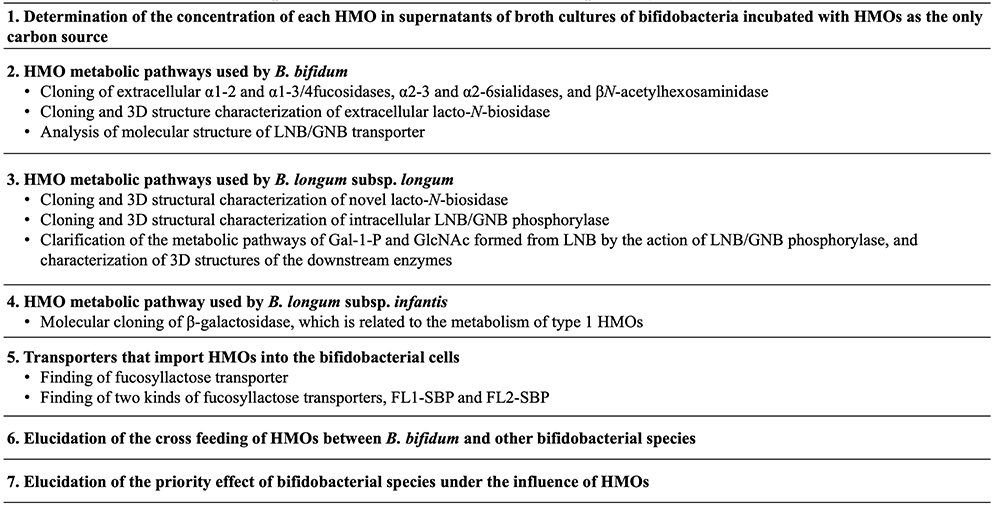
The pathway of HMO metabolism used by B. longum subsp. infantis, which has a high HMO assimilation ability, has been mainly elucidated by members of the Devid Mills group at California University of Davis. D.A. Sela et al. (2008) discovered HMO cluster-1, a large gene cluster, containing genes involved in HMO metabolism such as exo-glycosidases and transporters in the genome of the strain of B. longum subsp. infantis53. This pathway thus involves specific transporters to import intact HMOs into the cell and intracellular exo-glycosidases such as α-sialidase, α-fucosidase, β-galactosidase as well as β-N-acetylglucosamidase to hydrolyze HMOs, one by one, to monosaccharides. However, because the β-galactosidase, encoded in the HMO cluster-1, does not act on type 1 HMOs having LNB, which are more dominant in human milk than type 2 HMOs containing LacNAc, their study did not completely illustrate whole HMO metabolisms in this species. Erina Yoshida in Katayama’s group identified another β-galactosidase that can act on LNB and LNT, located outside of HMO cluster-154. As this β-galactosidase also can hydrolyze Galβ1-3Galβ1-4Glc (3’-GL) and Galβ1-3Galβ1-3Galβ1-4Glc (3’,3”-DGL), it is hypothesized that the specificity of the activity of this enzyme might have expanded between Galβ1-3Gal and Galβ1-3GlcNAc. As the result of these studies, most of the pathway of HMO metabolism used by B. longum subsp. infantis have been clarified, but the specificities of some transporters encoded in the HMO cluster-1 remains unclarified.
Asakuma et al. showed that B. longum subsp. longum had a low growth ability in in vitro experiment and consumed only LNT and LNFP- I44. Haruko Sakurama in Katayama’s group studied the ability of this species to metabolize LNT and LNFP- I by cloning the specific lacto-N-biosidase55. This lacto-N-biosidase had substrate specificity not only for LNT but also for LNFP- I and sialyllacto-N-tetraose a (Neu5Acα2-3Galβ1-3GlcNAcβ1-3Galβ1-4Glc, LST a); the specificity is different from that of another lacto-N-biosidase from B. bifidum. In addition, it should be noted that the expression of this enzyme requires the assistance of a specific chaperon.
To clarify totally the pathway of HMO metabolism used by bifidobacteria, substrate specificities of HMO transporters need to be characterized. Takahiro Matsuki (Yakult Honsha Co., Ltd.) identified a transporter specific for fucosyllactose (FL)56. They isolated 14 bifidobacterial strains, which could grow in the culture medium containing HMOs, and then selected specific strains that could grow in medium containing only FL. Then by exploring the genomes of these strains, they found the gene encoding the substrate binding protein, which was potentially responsible for import of FL, is located next to the genes encoding fucosidase and permease. Knock out of this gene in the strains of B. breve reduced the growth rate of this strain in culture medium containing HMOs relative to that of wild-type strain, indicating that this protein must be a FL transporter (FL-SBP). On the other hand, Mikiyasu Sakanaka in Katayama’s group (currently Ryuhkoku University) found that strains of B. longum subsp. infantis had two FL-SBP genes, named as “FL1-SBP” and “FL2-SBP”56. Growth rates were compared after knock out of either or both of them in the strains of this species and incubation of the strains in medium containing 2’-FL, 3-FL, LNFP- I or difucosyllactose [Fucα1-2Galβ1-4(Fucα1-3)Glc, DFL]57. It was shown that FL1-SBP knock out strain had lower growth rates than wild type strain when incubated with 2’-FL or 3-FL. On the other hand, FL2-SBP knock out strain had lower growth rates than wild type strain on DFL and LNFP- I. These data demonstrate that the substrate specificity of FL1-SBP is overlapping but distinct from that of FL2-SBP, and a subsequent structural analysis revealed its molecular basis by identifying the amino acid residues involved in the substrate recognition.
The crystal structures of GNB/LNB binding protein, two kinds of lacto-N-biosidase, GNB/LNB phosphorylase as well as the downstream enzymes, which are related to the metabolism of Gal-1-P or GlcNAc, were determined by the group of Shinya Fushinobu at Tokyo University58-63.
Although the four species of infant type bifidobacteria shown above have very a different ability to metabolize HMOs, they coexist in the colon. Miriam N. Ojima in Katayama’s group found that when two species of bifidobacteria were incubated in the culture containing HMOs, the species inoculated first grew predominantly by benefiting from cross-feeding, even if their ability to metabolize HMOs was low64. This effect, the so-called “priority effect” must be a hint to investigators interested in exploring the mechanism of the coexistence and the competition between some bifidobacterial species in the colon of breast-fed infants.
Since a few HiMOs are manufactured on an industrial scale, the numbers of in vitro and in vivo studies exploring the biological functions of HMOs have increased rapidly in the world. These studies have clarified the following functions; anti infection against pathogenic viruses or bacteria, immune modulation including anti-inflammation, prevention for NEC, which is a serious disease for preterm infants, activation of brain-nerve ability, and improvement of low body weight caused by malnutrition1. However, the number of published papers by Japanese scientists in this area is still small; this may be because of restriction on the use of experimental animals for in vivo study of food functions.
Intervention trials of infant formulas including 2’-FL, 2’-FL+LNnT, or 5 HMOs (2’-FL, 3’-SL, 6’-SL, LNT and 3-FL) have been performed to study the growth, health, and fecal condition of bottle-fed infants overseas65-68.
Cohort studies have also been performed to determine the relationship between the HMOs profile in breast milk and the frequency of development of NEC or infectious disease in breast-fed infants, or between the HMOs profile and development of cognitive ability in the infants. Few such cohort studies have been conducted in Japan, and the result of the first follow-up cohort study was published in 2024. After a cohort study was carried out by Tohoku Medical Megabank, Keigo Sato et al. (Meiji Co. Ltd.) determined the concentrations of 8 HMOs including 2’-FL, 3-FL, 3’-SL, 6’-SL, LST a, LST b (Galβ1-3[Neu5Acα2-6]GlcNAcβ1-3Galβ1-4Glc), LST c (Neu5Acα2-6Galβ1-4GlcNAcβ1-3Galβ1-4Glc), and disialyllacto-N-tetraose (Neu5Acα2-3Galβ1-3[Neu5Acα2-6]GlcNAcβ1-3Galβ1-4Glc, DSLNT) in 150 breast milk samples collected from the mothers in the cohort one month post-partum, and studied the correlation between each HMO concentration and the head circumference of the breast-fed infants, and that between this concentration and cognition scores on motor and socioemotional development tests, measuring communication skills, coarse movement skills, better movement skills, problem solving ability and personal social skills at 1, 5, and 9 months after birth69. Positive correlations were observed between the concentrations of 2’-FL and LST b in the milk and the head circumference and between the concentration of 2’-FL in the milk and cognitive ability. Only in the case of Sector donors of the mothers, the concentrations of 3’-SL and DSLNT in the milk were positively correlated with the score on a cognition ability test.
Similar cohort studies have been also performed in foreign countries. Berger et al. found a positive correlation between 2’-FL concentration in mothers’ milk at 1 month post-partum and cognitive score of infants, but did not find this correlation for 2’-FL concentration in mothers’ milk at 6 months post-partum70. Oliveros et al. showed a positive correlation between 6’-SL concentration in the mothers’ milk at 1 month post-partum and the cognitive and movement scores in breast-fed infants assessed at 6 and 18 months after birth, and a positive correlation between 2’-FL concentration and the movement in infants assessed at 6 months71. Cho et al. found that 3’-SL concentration in milk of mothers who presumably have blood group type A and whose milk contained A tetrasaccharide (GalNAcα1-3[Fucα1-2]Galβ1-4Glc) positively correlated with the composite score for listening and spoken language in breast-fed infants72. Mansell et al. described a positive correlation between 2’-FL concentration in mothers’ milk at around 6 weeks post-partum and head circumference of the breast-fed infants at 12 months - 4 years of age73. Wejryd et al. carried out an explorative trial to find the correlation between the concentrations of 15 HMOs in mothers’ milk and the neurodevelopment of preterm infants at 2 years of age whose body weights were less than 1000 grams at birth. Results revealed associations between specific HMOs and neurodevelopmental outcome: 3-FL was associated with less neurodevelopmental impairment, LST b with higher cognitive score, and LST a with worse neurodevelopmental impairment outcome74.
Regarding childhood obesity, one should pay attention to adiposity rebound (AR), which is the phenomenon of decreased body mass index (BMI) in early childhood and then rebound in BMI at age 5-6 years. Early AR , occurred before 5 years of age, is associated with higher risk of lifelong obesity and metabolic disorders and may be influenced by breastfeeding. Kento Sawane et al. (Ezaki Glico Co., Ltd) with Tohoku Medical Megabank Organization studied the potential correlation between AR status and the concentrations of 15 HMOs in milks collected 1 month post-partum in a case-control study of 184 mother-child pairs (93 AR cases, 91 controls). Analyses were stratified by maternal secretor status (secretor or nonsecretor). The results showed an inverse association of early AR with α-diversity, sialic acid-bound HMOs, and 3’-SL, and a tend toward interaction between LST a and maternal secretor status regarding AR75.
As there are still few examples of such cohort studies, further studies should be performed to explorer the relationship between each HMO concentration in mothers’ milk and the development of cognitive ability or AR status in breast-fed infants. The above findings should only be interpreted as a foundation for future hypothesis-driven research.
This minireview introduced the first-in-the-world studies by Kobata et al. and Kitagawa et al. that led to the structural characterization of HMOs. A Japanese company was the first in the world to add sialyllactoses, separated from cheese whey, to infant formula, and Japanese scientists pioneered plant scale production of 3’-SL by the cell reaction method using recombinant bacteria. Kitaoka and Katayama have made a large contribution to clarification of the HMO metabolic pathways used by infant type bifidobacteria.
Although a few HiMOs have been commercialized and added to infant formula in many overseas countries, their commercial utilization has been banned in Japan. The examples of in vitro and in vivo studies to clarify biological functions, and the cohort study on HMOs, are much smaller in number in Japan than in other countries. The invention trial process for HiMOs has never been started in Japan.
The commercial base utilization of HiMOs may be achieved by complying with legal regulations in Japan in the future, and these products may become additives to infant formula and functional foods as in many overseas countries. As for the practical utilization of HiMOs, more functional, clinical and cohort studies as well as intervention trials should be performed. Because the numbers of commercially available HiMOs are still small, the effort to develop a method of producing additional HiMOs with larger molecular weight should be continued.