
Bryan P. Toole
Bryan Toole was educated in Melbourne, Australia, where he received a B.Sc. degree from the University of Melbourne and a Ph.D. in Biochemistry at Monash University. His Ph.D. work in the laboratory of Professor Dennis Lowther included the initial isolation and purification of the dermatan sulfate proteoglycan, now named decorin, and the first demonstration that decorin interacts with collagen under physiological conditions. In 1968, he moved to Boston for postdoctoral studies at Massachusetts General Hospital and Harvard Medical School in the laboratory of Dr. Jerome Gross. There Dr. Toole showed that hyaluronan surrounds and interacts with motile and proliferating cells during regeneration and embryonic development. In 1972, he joined the Harvard University faculty and established his own laboratory dedicated to the study of hyaluronan-cell interactions in embryonic development and cancer and to the biochemical characterization of hyaluronan receptors. In 1980, he joined the Tufts University Health Sciences Campus in Boston as Professor of Anatomy and Cellular Biology and from 1985 to 1992 was Chair of this Department. He is now the George Bates Professor of Histology and Director of the Ph.D. Program in Cell, Molecular and Developmental Biology at the Tufts Health Sciences Campus. His laboratory continues to focus on hyaluronan-cell interactions in morphogenesis and cancer, as well as on the role of tumor-stromal cell interactions in the regulation of matrix metalloproteinases during metastasis.
Almost everything about hyaluronan is unusual; yet its extraordinary attributes derive from a chemical composition that is very simple. Hyaluronan is a uniformly repetitive, linear glycosaminoglycan composed of 2,000-25,000 disaccharides of glucuronic acid and N-acetylglucosamine:
[beta-1,4-GlcUA-beta-1,3-GlcNAc-]n
Despite this apparent chemical simplicity, hyaluronan is unlike other glycosaminoglycans in its mechanism of synthesis, its size, and its physical properties. These properties of hyaluronan have already been discussed in previous articles in this series. Another uniquely important aspect of hyaluronan biology, namely, its effects on cell behavior, will be the subject of this article.
For several decades after the discovery of hyaluronan, it was assumed that its major functions were in the biophysical and homeostatic properties of tissues. However, studies in the 1970s of dynamic cellular systems such as embryonic development, tissue regeneration, and tumorigenesis led to our current understanding that hyaluronan also plays a crucial role in cell behavior1,2. In these early studies, it was found that cells divide and migrate within an extracellular matrix that is rich in hyaluronan and that dramatic modulations in hyaluronan concentration and organization accompany the cellular changes that take place as tissues and organs differentiate. Striking examples of dynamic events during which cells are surrounded by hyaluronan-rich matrices are mesenchymal cells invading the primary corneal stroma to form the mature cornea; neural crest cells traveling from the neural tube to form ganglia of the peripheral nervous system; sclerotomal cells approaching and surrounding the notochord to form vertebrae; cushion cells migrating from the endocardium towards the myocardium during formation of heart valves; neuronal and glial precursors moving and proliferating during brain development; mesenchymal cells dividing and migrating during embryonic limb development, salamander limb regeneration, tendon regeneration, and fetal wound repair; and tumor cell growth and invasion1,2. Cellular proliferation and migration in these systems usually leads to assembly of cells in appropriate numbers and positions prior to overt differentiation to tissues and organs. At this stage of morphogenesis, pericellular hyaluronan often decreases in concentration and rearranges in such a way as to allow or promote initiation of cell interactions essential for subsequent differentiation, e.g., during cellular condensation prior to formation of muscle and cartilage in the embryonic limb (Section VI).
One way in which hyaluronan facilitates cell migration is by creating hydrated pathways that allow cellular or fibrous barriers to be penetrated by cells. Formation of hydrated pericellular matrices may also facilitate cell rounding during mitosis. A subsequent decrease in hyaluronan concentration leads to decreased volume of intercellular matrix and closer apposition of cells prior to differentiation. In addition to these physicochemical effects, however, the studies noted above also led to the discovery of hyaluronan receptors on the surface of embryonic and tumor cells and to the enormous proliferation of modern studies that have demonstrated definitively that hyaluronan exerts a direct and profound effect on cell behavior1-3.
The early work on embryonic development and tissue remodeling mentioned above suggested strongly that hyaluronan directly influences cell behavior. Consequently, evidence was sought and obtained for the presence of hyaluronan receptors on the surface of cells. Subsequent investigations led to the full molecular characterization of two classes of cell surface hyaluronan receptors, namely, CD44 and RHAMM2-5, which are described briefly belowa.
a Reviews by Cheryl Knudson on CD44 and by Eva Turley on RHAMM will appear later in this series.
CD44 is a widely distributed cell surface glycoprotein that is encoded by a single gene but expressed as numerous isoforms as a result of alternative splicing. The simplest and most widespread is termed the standard isoform and is denoted CD44s (often alternatively termed the hematopoietic isoform or CD44H). CD44s contains transmembrane, cytoplasmic, and extracellular regions that are common to all membrane-bound isoforms of CD44 (Fig. 1).
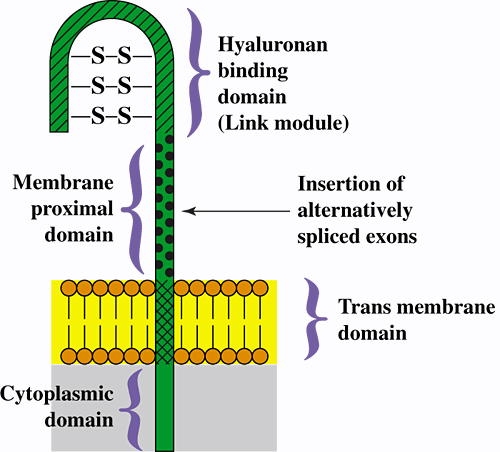
Fig. 1 Model for the structure of CD44, showing the protein domains for the standard isoform of CD44.
The products of various combinations of ~10 alternatively spliced variant exons are inserted at the position indicated by the arrow, giving rise to numerous variant isoforms of CD44. The extracellular domains of CD44 are highly, but variably, glycosylated, and several serine residues in the cytoplasmic domain can be phosphorylated.
The extracellular region of CD44s includes two major domains: (1) The amino-terminal domain is a "link module" similar to that found in many hyaluronan-binding proteins. This domain is the presumed binding site for hyaluronan, although binding is subject to numerous positive and negative influences from other regions of the molecule, e.g., glycosylation, alternative splicing, dimerization, clustering in the plasma membrane, and integrity of the cytoplasmic domain4. (2) The so-called membrane-proximal domain lies between the hyaluronan-binding and transmembrane domains. Various combinations of the products of approximately 10 variant exons can be spliced into a single position within the membrane-proximal domain (see Fig. 1) to give rise to numerous variant isoforms of CD44 that exhibit different physiological properties and variable ability to bind hyaluronan.
Alternative splicing also generates several isoforms of RHAMM, including intracellular and cell surface isoforms. However, RHAMM does not contain a "link module" domain but does include two regions that contain a potential hyaluronan-binding "motif," namely, the linear sequence B(X7)B, where B is a basic amino acid residue and X is any non-acidic amino acid. This sequence is present in most hyaluronan-binding proteins and, at least in part, accounts for their hyaluronan-binding capability5.
Since interactions between cell-surface hyaluronan and CD44 or RHAMM mediate many cellular effects of hyaluronan, the biochemical mechanisms by which these interactions are transduced into intracellular signals that bring about these effects are now being intensely studied by several groups. Although many promising observations have been made, there is little consensus with respect to the signaling pathways or cytoskeletal rearrangements initiated by these interactions at this time.
Several cell types exhibit highly hydrated, hyaluronan-dependent pericellular matrices or "coats." In culture, these matrices are difficult to visualize by conventional light microscopy. However, the coats can easily be visualized indirectly by exclusion of particles and are usually 5-10 micrometers in thickness (Fig. 2).
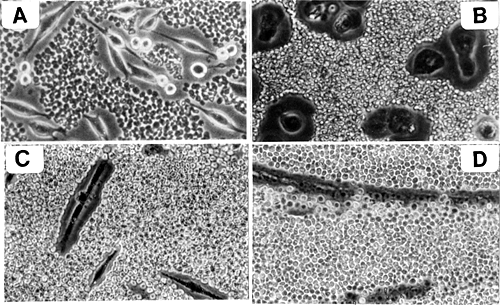
Fig. 2 Hyaluronan-dependent pericellular matrices.
Examples of the large pericellular matrices surrounding rat fibrosarcoma cells (A), chick embryo chondrocytes (B) and chick embryo myoblasts (C) are shown. The matrices have been visualized by exclusion of particles (fixed red blood cells). Treatment of these cells with hyaluronan-specific hyaluronidase removes the matrices (not shown). Panels C and D show the loss of pericellular matrix that occurs during muscle development when myoblasts (C) fuse to form myotubes (D).
These pericellular matrices provide the milieu in which numerous cellular activities take place and influence the behavior of cells in many circumstances. For example, during tissue formation or remodeling, such matrices can provide a highly hydrated, fluid pericellular environment in which assembly of other matrix components and presentation of growth and differentiation factors can occur without interference from the highly structured fibrous matrix usually found in fully differentiated tissues. Thus embryonic mesenchymal cells, including the precursors of muscle and cartilage, embryonic glial cells, neural crest cells, and even some embryonic epithelial cells, exhibit prominent pericellular matrices. In some cases, such as in cartilage, the pericellular matrix is a unique structural component that protects the cells and contributes to the characteristic properties of the differentiated tissue.
The function and assembly of pericellular matrices have been studied extensively, especially for chondrocytes, and have been shown to depend on three features6,7. (1) Their integrity is dependent on hyaluronan. Thus, treatment of cells exhibiting pericellular matrices with hyaluronan-specific hyaluronidase destroys their structure. (2) The assembly and density of the pericellular matrix of chondrocytes depend on a specific interaction of hyaluronan with the proteoglycan aggrecan. (3) Hyaluronan must be tethered to the cell surface. Tethering of hyaluronan to different cell types can occur by two independent mechanisms, i.e., by binding to specific hyaluronan receptors (e.g., CD44) on the cell surface (Fig. 3A) or by transmembrane interaction of "nascent" hyaluronan with hyaluronan synthase or associated proteins on the cytoplasmic face of the plasma membrane (Fig. 3B). In the case of chondrocytes, tethering is mediated mainly by the interaction of hyaluronan with CD44.
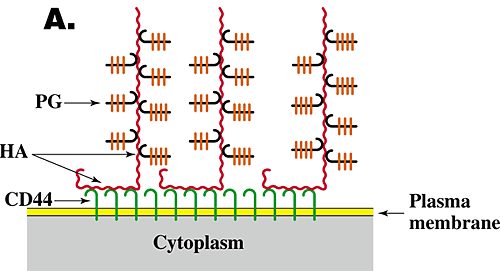
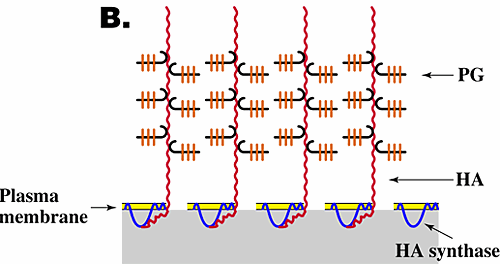
Fig. 3 Models for the structure of hyaluronan-dependent pericellular matrices.
As discussed in the text, coat formation usually requires that hyaluronan is tethered to the cell surface and proteoglycan is bound to the hyaluronan. Tethering of hyaluronan to the cell surface can occur by binding to cell surface receptors such as CD44 (A) or by transmembrane interaction with hyaluronan synthase (B). HA, hyaluronan; PG, proteoglycan.
In many embryonic cells that exhibit pericellular matrices, hyaluronan is most likely tethered by sustained attachment to hyaluronan synthase. Indeed, transfection of cells lacking pericellular matrices with hyaluronan synthase is in some cases sufficient to induce matrix formation. However, it is not clear whether hyaluronan tethered to synthase produces a pericellular matrix without also binding to proteoglycan. Theoretically, if hyaluronan molecules of high enough density were tethered at the cell surface, they would form an extended array or "brush" that would protrude outwards from the cell surface and form a continuous network via self-interactions; this arrangement may then be sufficient to form such a matrix. It is clear, however, that addition of proteoglycans favors pericellular matrix formation by increasing matrix density and thus possibly matrix stability7. Inclusion of other hyaluronan-binding proteins such as inter-alpha-trypsin inhibitor and tumor necrosis factor-stimulated gene 6 (TSG-6) may also stabilize pericellular matrices by cross-linking hyaluronan chains.
The matrices that surround migrating and proliferating cells during morphogenesis of embryonic organs, during regeneration and healing, or during pathological processes such as tumorigenesis become enriched in hyaluronan and resemble the pericellular matrices described in the previous section. In what ways might hyaluronan affect these cellular events? Many studies using in vitro and in vivo systems have shed some light on this question, albeit still a somewhat feeble light at this point.
An important way in which a hyaluronan-rich matrix could promote cell proliferation is by providing a hydrated pericellular zone that facilitates cell rounding during mitosis. Hyaluronan synthase activity has been shown to fluctuate with the cell cycle and to peak at mitosis. Thus, extrusion of hyaluronan onto the cell surface at mitosis would create a hydrated micro-environment that promotes partial detachment and rounding of the dividing cells. In support of this idea, inhibition of hyaluronan synthesis has been shown to lead to cell cycle arrest at mitosis, just before cell rounding and detachment8.
As mentioned in Section II, hyaluronan-rich matrices create hydrated pathways that separate cellular or fibrous barriers to penetration by the invading cells. In addition, modulation of the density of these matrices by variation in the degree of binding of proteoglycans to pericellular hyaluronan may regulate cessation of migration. For example, sulfated proteoglycans act as barriers to neural crest cell migration and neurite outgrowth in vitro and in vivo. In neural crest cells, this inhibition is dependent on the interaction of proteoglycan with cell surface hyaluronan, which would in turn increase the density of the pericellular matrix, thus transforming the pericellular matrix from being conducive to cell migration to being inhibitory6.
As stated earlier, pericellular hyaluronan often decreases in concentration along with changes in its interaction with the cell surface after assembly of cells in the correct number and place by proliferation and migration. This transition sometimes actively promotes initiation of cell interactions essential for differentiation. Hyaluronan-induced cell aggregation was first observed in experiments in which the addition of small amounts of hyaluronan to lymphoma cells or macrophages caused aggregation of these cells and in which either removal of endogenous hyaluronan from the surface of transformed fibroblasts or addition of high concentrations of exogenous hyaluronan to these cells inhibited their ability to aggregate. These findings were interpreted to mean that cell surface hyaluronan (whether endogenous or exogenously added) could cross-bridge cells via interaction with receptors on adjacent cells. Removal of this hyaluronan would thus block aggregation. Addition of excess hyaluronan would cause occupation of all receptors by hyaluronan, thus also blocking cross-bridging (Fig. 4)1,2. Hyaluronan-induced cell aggregation mediates initial formation of cell condensates during differentiation of several embryonic tissues (see Section VI and Fig. 5).
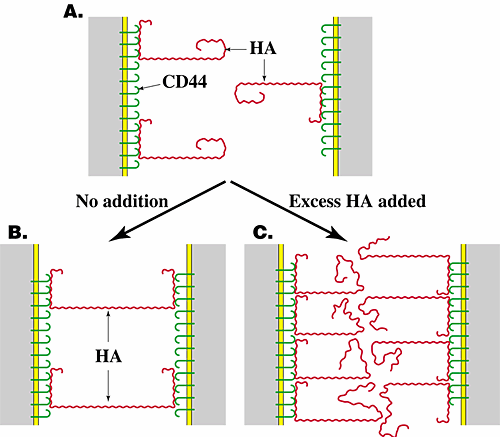
Fig. 4 Hyaluronan-mediated cell aggregation.
Hyaluronan cross-bridges cells bearing hyaluronan receptors such as CD44, causing cell aggregation (B). If the cells are treated with hyaluronidase (not shown) or with excess hyaluronan (C), aggregation is inhibited. HA, hyaluronan.
In addition to providing a suitable hydrated milieu or to cross-bridging cells, interaction of hyaluronan with its cell surface receptors also initiates signaling pathways that promote cell movement, proliferation, or differentiation. Several investigators have demonstrated that cell movement in vitro is promoted in the presence of hyaluronan, that invasion into three-dimensional collagen gels is dependent on hyaluronan synthesis, and that cell movement is inhibited as a result of degrading the hyaluronan itself or of blocking the binding of hyaluronan to either of its receptors (CD44 or RHAMM). Interaction of hyaluronan with RHAMM stimulates tyrosine phosphorylation of several proteins, including a key component of focal adhesions, p125FAK, resulting in regulation of focal adhesion turnover and promotion of cell motility. Interaction of hyaluronan with cell surface CD44 also stimulates cell migration in some tumor cell types, e.g., glioma and melanoma cells. Thus it seems that interaction of hyaluronan with either receptor can stimulate cell movement, but their relative importance may depend on the cell type or other physiological factors. Similarly, interaction of hyaluronan with CD44 or RHAMM stimulates signaling pathways involved in cell proliferation in different cell types. Nevertheless, the details of these putative pathways are by no means clear for either cell proliferation or locomotion.
Studies carried out on embryonic limb development provide an illustrative example of the way in which modulation of pericellular hyaluronan concentration and organization influences several of the events leading to differentiation in vivo (Fig. 5)2,9. First, the volume of hyaluronan-rich matrix separating cells at different stages of limb development closely parallels progressive steps in differentiation. Early limb mesodermal cells are surrounded and separated by extensive, hyaluronan-enriched matrix in vivo (Fig. 5A) and express voluminous hyaluronan-dependent pericellular matrices in culture. At this stage, pericellular hyaluronan appears to be tethered to the cell via transmembrane interaction with hyaluronan synthase (see Fig. 3B) since it is retained at the cell surface in a nonreceptor-mediated manner. The pericellular matrix maintains separation of early mesodermal cells, consistent with the predicted behavior of surface-associated polymers, i.e., due to their constant motion, polymer molecules on apposing surfaces do not interdigitate7. This hydrated pericellular matrix would facilitate proliferation and migration of early mesenchymal precursors of limb tissues, as discussed in the preceding section. This process has been demonstrated directly in the case of muscle differentiation, where it has been shown that continued exposure of myoblasts to hyaluronan supports proliferation and migration but inhibits differentiation.
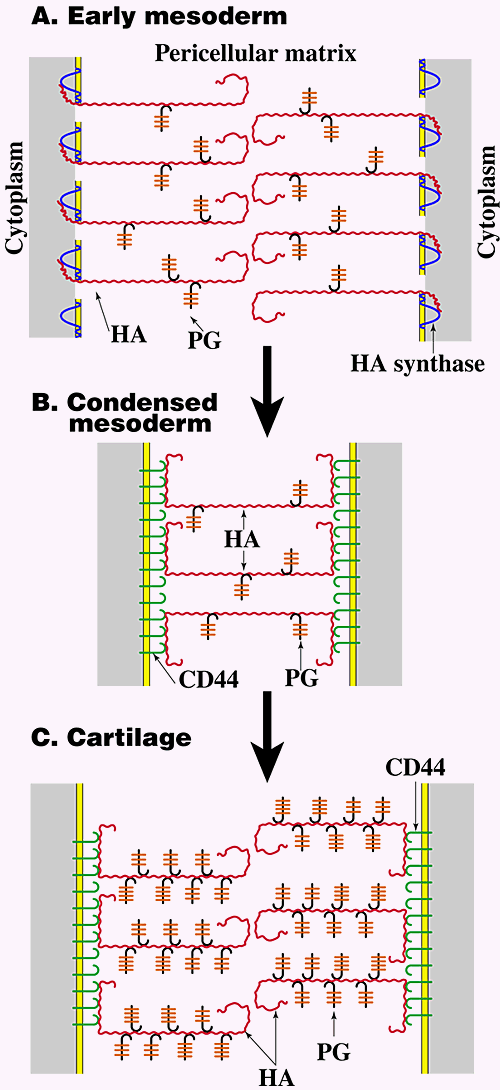
Fig. 5 Hyaluronan-cell interactions during embryonic limb development.
As discussed in the text, early mesodermal cells (A) are surrounded by large pericellular matrices in which the hyaluronan is most likely tethered to the cell surface via transmembrane interaction with hyaluronan synthase (see Fig. 3B). At this stage the proteoglycan in the pericellular matrix is mainly versican and is present at a relatively low concentration. During mesodermal condensation prior to cartilage and muscle differentiation (B), lysosomal hyaluronidase levels increase, intercellular hyaluronan concentration drops markedly, hyaluronan receptors (most likely CD44) appear on the surface of the mesodermal cells, and cell surface hyaluronan cross-bridges the cells in the condensate via multivalent interaction with receptors on adjacent cells (see Fig. 4B). During cartilage differentiation (C), hyaluronan remains bound to CD44, but the concentration of proteoglycan, now mainly aggrecan, increases dramatically and large pericellular matrices re-form in the manner shown in Fig. 3A. HA, hyaluronan; PG, proteoglycan.
Subsequent to the early stage of limb development described in the previous paragraph, the mesoderm condenses, i.e., the intercellular matrix decreases in volume, at sites of future cartilage and muscle differentiation. This state is paralleled by loss of ability of the mesodermal cells to form hydrated pericellular matrices in culture. During this condensation, the level of lysosomal hyaluronidase in the mesodermal cells increases, and much of the hyaluronan is removed from the intercellular matrix, thus accounting for the decreased intercellular volume. However, hyaluronan continues to be retained at the cell surface, but it is now retained via interaction with receptors that appear on the mesodermal cells. This cell surface hyaluronan interacts multivalently with receptors on adjacent cells, thus cross-bridging them within the condensate (Fig. 5B). Cross-bridging occurs in an analogous fashion to that discussed in the preceding section (Fig. 4). Similar events comprise an early step in mesodermal condensation in other developing tissues also, e.g., skin and teeth.
Further differentiation of condensed limb mesoderm to cartilage (Fig. 5C) is accompanied by extensive matrix formation in vivo and recovery of the ability of the mesodermal cells to form extensive hyaluronan-dependent pericellular matrices in culture. In these matrices, however, hyaluronan is tethered to the cell surface by interaction with CD44 (see Fig. 3A), and the proteoglycan concentration is much higher than in the matrices surrounding early mesodermal cells. Thus, the pericellular matrix of differentiated chondrocytes would be denser than that of early mesodermal cells, reflecting its structural rather than morphogenetic role.
During differentiation of cartilage to long bones, lacunae surrounding hypertrophic chondrocytes are highly enriched in hyaluronan, and the swelling pressure exerted by this hyaluronan causes expansion of lacunae as bone growth occurs. Subsequently, in the zone of erosion, the hyaluronan within these lacunae is removed via CD44-mediated endocytosis10. CD44-mediated internalization of hyaluronan is an important step in differentiation of several other tissues also, e.g., dermis and lung.
With respect to muscle differentiation, mononucleated myoblasts are also initially kept separated from each other by a hyaluronan-rich matrix in vivo, and they express large pericellular matrices in culture. However, during the processes of condensation and fusion to give myotubes, these pericellular coats are lost (Fig. 2C, D), thus allowing the intimate cell interactions required for subsequent differentiation.
Most malignant solid tumors contain elevated levels of hyaluronan, and these high levels of hyaluronan expression correlate with poor differentiation and decreased survival rates in some human carcinomas. Enrichment of hyaluronan in tumors may be caused by increased hyaluronan production by tumor cells themselves or by interactions between tumor cells and surrounding stromal cells that induce increased hyaluronan production by the latter11.
Direct experimental evidence has been obtained implicating hyaluronan and hyaluronan receptors in tumor growth and metastasis for several types of cancers3,12-14. However, the mechanisms whereby hyaluronan-receptor interactions influence tumor cell behavior are not clearly understood, and this is currently a very active area of investigation. Despite this lack of detailed understanding, it is evident that manipulation of hyaluronan interactions leads to impressive inhibition of growth or metastasis of several tumor types. Antibodies to CD44, soluble forms of CD44 or RHAMM, hyaluronidases, and oligomers of hyaluronan have all been used effectively to inhibit tumor growth or metastasis in animal models. For example: (1) Administration of hyaluronan oligosaccharides, which would be expected to antagonize interactions of hyaluronan polymer with CD44 or RHAMM, decreases murine melanoma growth in vivo12. (2) Ex vivo treatment of murine fibrosarcoma cells with soluble RHAMM in culture leads to reduced tumor growth and metastasis when these cells are implanted in vivo13. (3) Transfection of murine mammary carcinoma cells with soluble forms of CD44 leads to tumor cell apoptosis in vivo and completely inhibits formation of metastatic nodules in the lung; this inhibition does not occur when a mutated form of soluble CD44, which does not bind hyaluronan, is used for transfection14.
It is now fully apparent that hyaluronan plays a central role in the physical and chemical properties of pericellular milieux as well as extracellular matrices. The network-forming, visco-elastic and charge characteristics of hyaluronan are fundamental to the biomechanical properties and homeostasis of many tissues, and specific interactions of hyaluronan with proteins and proteoglycans are intrinsic to the structural properties of extracellular matrices. Now, however, it is also appreciated that hyaluronan is a crucial pericellular and cell surface constituent that, through interactions with other macromolecules, participates importantly in regulating cell behavior during numerous morphogenetic, restorative and pathological processes.
Research in these cellular areas is proceeding rapidly. Of special interest is ongoing work on: (1) the part played by hyaluronan synthases in regulating the properties of pericellular milieux during dynamic cellular processes, as well as the coordination between synthase activity and the intracellular signaling pathways governing these processes; (2) the regulation and importance of hyaluronan polymer size in various cellular events; (3) the involvement of cell surface hyaluronan receptors, such as CD44 and RHAMM, in transmitting signals between the extracellular and intracellular compartments, and the role of these signals in cell behavior; and (4) manipulations of hyaluronan-cell interactions as therapeutic interventions in disease.