Yasuhiro Shimizu MD, PhD
Department of Gastroenterological Surgery, Yokohama City University Graduate School of Medicine. A researcher in Division of Cancer Immunotherapy, Exploratory Oncology Research & Clinical Trial Center, National Cancer Center since April 2017.

Tetsuya Nakatsura MD, PhD
Chief, Division of Cancer Immunotherapy, Exploratory Oncology Research & Clinical Trial Center, National Cancer Center
Graduated from Kumamoto University Medical School in 1992. Specialty is tumor immunology. As a former surgeon researcher, Dr, Nakatsura would like to realize the development of immunotherapy and immune-prevention methods of cancer patients who cannot be cured only by surgery.
In addition to surgery, radiation therapy, and chemotherapy, there are high hopes for a fourth modality of cancer treatment: immunotherapy, which exploits the physiological immune system. Emerging immunotherapies that block immune checkpoints, including antibodies to CTLA-4, PD-1, PD-L1, and similar molecules, have been shown to have dramatic, long-term antitumor effects 1,2. Tasuku Honjo and James P. Allison were awarded the 2018 Nobel Prize in physiology for their research on these drugs. In cancer immunotherapy, identification of improved cancer-specific antigens is essential for higher effectiveness and lower side effects. Since Boon first identified the cancer-specific antigen for melanoma in 1991, many researchers throughout the world have sought new cancer antigens 3. Our research group identified glypican-3 (GPC3), a hepatocellular carcinoma specific antigen, in 2001. GPC3 has high cancer specificity and is an ideal target for cancer immunotherapy. This article is an overview of GPC3 and cancer immunotherapy targeting GPC3.
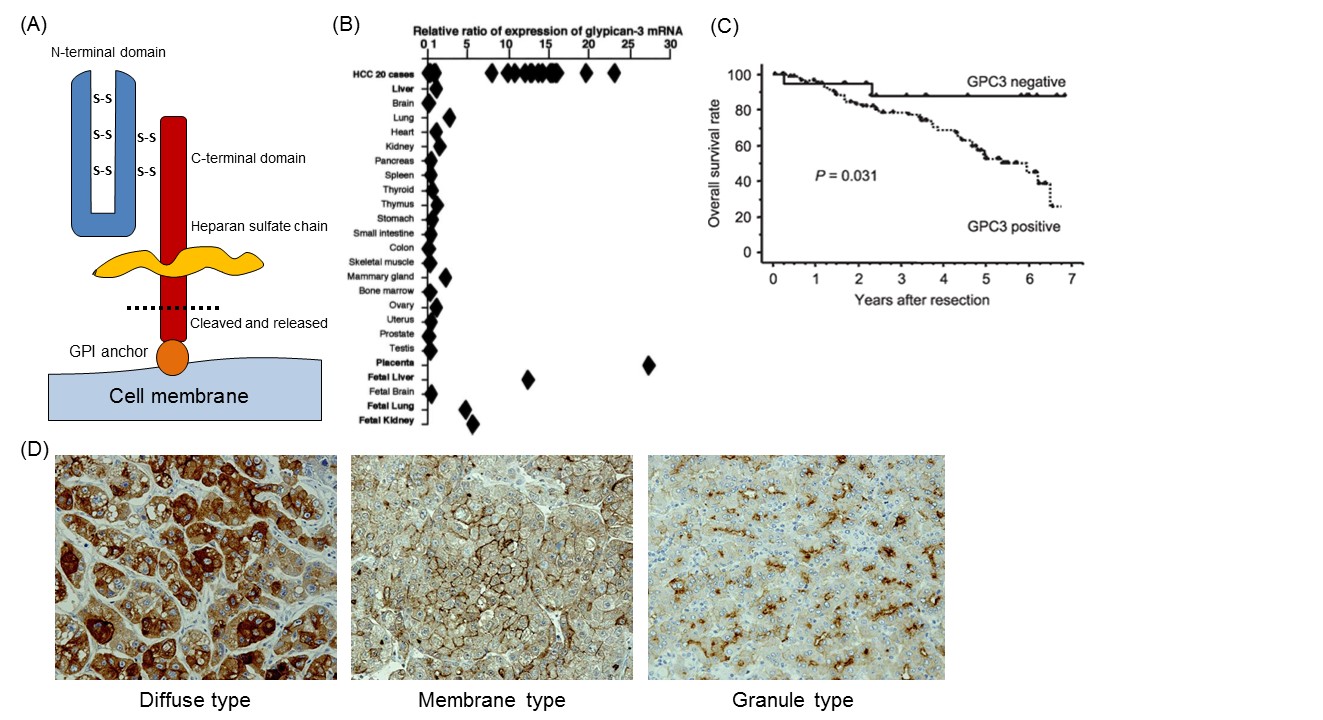
We identified GPC3 as a cancer-specific antigen in a search to discover candidates for novel cancer antigens from the cDNA microarray data of several tens of thousands of genes in various organs, including cancerous and noncancerous parts 4. GPC3, a 65-kDa protein consisting of 580 amino acids, is a member of the GPC family that is a heparan sulfate chain proteoglycan bound to the cell membrane by a glycosylphosphatidylinositol (GPI) anchor (Fig. 1A) 5. GPC3 regulates the cell proliferation signals by binding growth factors such as Wnt, fibroblast growth factor, and IGF, and plays an important role in proliferation and differentiation of embryonic cells. In addition, its gene is present in the X chromosome (Xq26) with high homology between human and mouse. Mutations and deletions of this gene cause gigantism with various malformations, e.g., Simpson-Golabi-Behmel syndrome, in humans as well as in knockout mice 6. GPC3 is expressed in various fetal tissues (liver, lung, kidney and placenta), but not observed in postnatal normal tissues due to suppression by DNA methylation within the promoter region. On the other hand, it is expressed specifically in hepatocellular carcinoma (HCC), melanomas, ovarian clear cell carcinoma (OCCC), lung squamous cell carcinomas, and certain childhood cancers (hepatoblastomas, nephroblastomas and yolk sac tumors). Notably in HCC, it is expressed in approximately 80% or more of the whole carcinoma derived from either hepatitis B or C (Fig. 1B) 4, 7, 8. The function of membrane-anchored GPC3 in these cancers is unknown, but it is almost certainly involved in neoplastic transformation in HCC (Fig. 1C) 8. Additionally, immunohistochemical analysis of HCC tissues suggested the existence of a number of GPC3 location types that we classified into three categories: a type in which GPC3 is stained diffusely throughout the cells (diffuse type), centrally on the cell membranes (membrane type) and a third type that is granular in form (granule type) (Fig. 1D).
As described above, GPC3 is a protein bound to the cell membrane by a GPI anchor that is cleaved and secreted into the blood (Fig. 1A). The mammalian GPC family is cleaved at the GPI anchor level by endogenous GPI phospholipase D 9. It was formerly thought that Notum (a secretory feedback inhibitory protein of the Wnt signal preserved in many organic species) cleaved the GPI anchor within the GPC protein, like a phospholipase, and pulled away the complex of GPC and Wnt from the cell surface to inhibit the Wnt pathway, but it is now reported to control the signal without cutting off the anchor 10,11. Moreover, it is believed that GPC3 is cleaved between Arg358 and Ser359 and the N-terminal region is released as a soluble protein from the cancer tissues and circulates in the blood 12. Therefore, it seems that various forms of GPC3 protein circulate in the blood, but the functions of these GPC3 proteins in the blood are not clear.
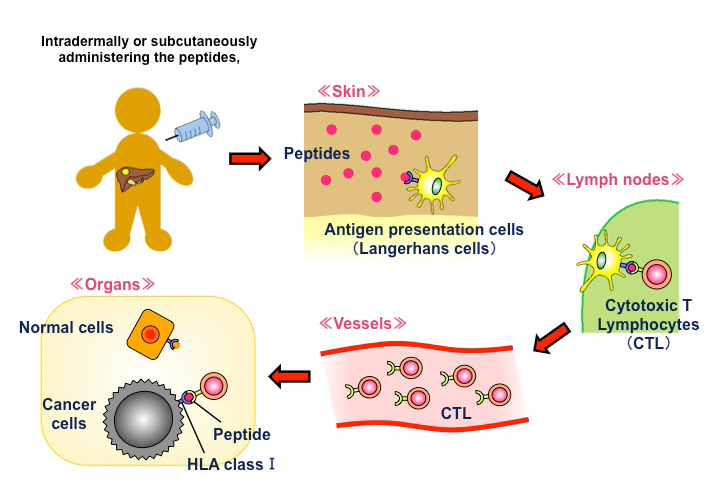
The above characteristics render GPC3 an ideal target for cancer immunotherapy. We are developing some immunotherapies targeting GPC3, one of which is cancer peptide vaccine therapy (Fig. 2). The GPC3 peptides in development as cancer vaccines are restricted to HLA-A24 and HLA-A2. Of note, the former is present in approximately 60% of Japanese individuals, while the latter is present in 40% of Japanese and is also a major haplotype in Caucasians. We have performed some clinical trials of these peptide vaccines 13. These trials not only provided evidence of the safety of the vaccines but also demonstrated that peptide-specific cytotoxic T cells (CTLs) are induced in blood and cancer tissues in vaccine-administered patients, confirming immunological efficacy. We also observed cases with good clinical responses in patients with advanced HCC and OCCC and five cases of 4-year survival without recurrence in children with refractory hepatoblastoma. Based on the findings of these trials, we are currently developing new immunotherapies that can evoke further clinical effects 13.
As previously described, GPC3 is subject to shedding into serum in HCC patients. Its effectiveness as a tumor marker has been reported 14,15. We constructed a novel sandwich ELISA system that can predict HCC recurrence after surgery. Significant recurrence manifested in patients with high levels of postoperative serum GPC3 16. We noted in immunohistochemical staining of some GPC3-positive HCC that a few surrounding normal cells weakly expressed GPC3, indicating that these cells may also be involved in postoperative GPC3 secretion and recurrence 16. In partnership with a private company, we have developed an assay to quantify serum full-length GPC3, which is considered to be the most physiologically important. By combining serum GPC3 and existing tumor markers, such as Alpha-Fetoprotein and Protein Induced by Vitamin K absence-II, we succeeded in predicting early recurrence of postoperative hepatocellular carcinoma (manuscript in preparation). We are also conducting a prospective study demonstrating the use of this assay as an indicator and predictor of hepatocellular carcinogenesis in patients with chronic hepatitis and cirrhosis. Work is in progress investigating the value of this assay in early diagnosis as well as in prediction and assessment of response to anti-GPC3 therapy.
GPC3 has shown unprecedented cancer specificity and is being studied as a target for cancer immunotherapy worldwide. However, much remains unclear about its natural history, function and dynamics. Wider knowledge of the nature of GPC3 is required for the development of more effective treatments.
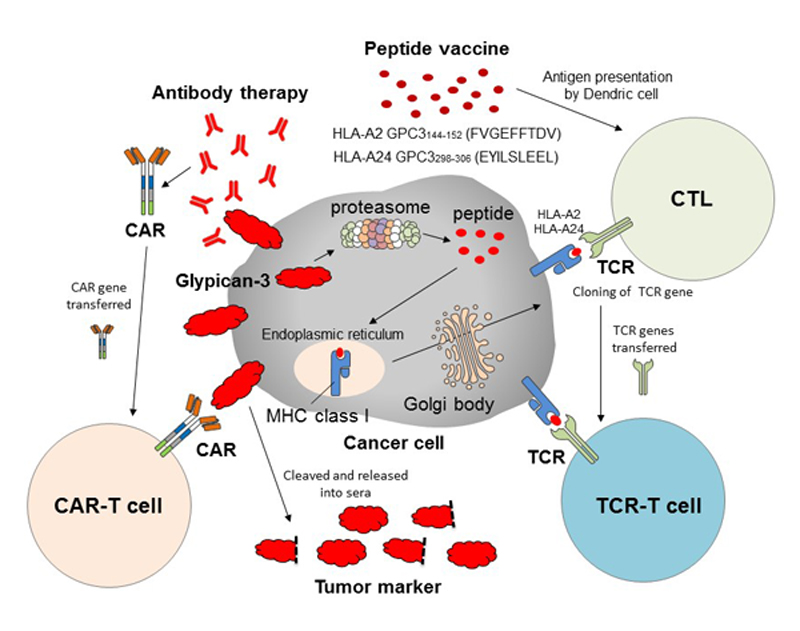
The development of cancer immunotherapy targeting GPC3 has entered a new era (Fig. 3). In addition to peptide vaccine therapy, clinical trials of antibody therapy (GC33) that specifically recognizes membrane-binding GPC3 are ongoing. Recently, GPC3-specific chimeric antigen receptor gene-transduced T cell (CAR-T) therapy using the antibody was developed, and clinical trials of the novel cells are ongoing in the United States and China. We are now developing adoptive immunotherapies using CAR-T and T cells transduced with T cell receptors of the best CTL clones obtained from our trials. Research on therapies for patients with GPC3-positive cancer with high malignancy potential continues apace.