
Tomohiko Fukuda
Present post: Doctor of Agriculture, Associate Professor, Division of Regulatory Glycobiology, Faculty of Pharmaceutical Sciences, Tohoku Medical and Pharmaceutical University
Biography: Graduated from the Doctoral course at the Graduate School of Agriculture, Osaka Prefecture University in 1995 (Professor Yoshio Imai).
Since then, he has been involved in research on the drug metabolizing enzyme Cytochrome P450 and extracellular matrix as a Research Fellow at the Research Institute, Osaka Medical Center for Maternal and Child Health and as a researcher of the ERATO Sekiguchi Biomatrix Signaling Project (Director Kiyotoshi Sekiguchi). Since 2006, he has been conducting research on the understanding of the function of N-glycans, especially core fucose at the laboratory of Professor Jianguo Gu in Tohoku Pharmaceutical University (currently Tohoku Medical and Pharmaceutical University).

Jianguo Gu
Present post: Doctor of Medicine, Professor, Division of Regulatory Glycobiology, Faculty of Pharmaceutical Sciences, Tohoku Medical and Pharmaceutical University
Biography: Graduated from the Doctoral course of Physiology, Faculty of Medicine, Osaka University in 1993 (Professor Naoyuki Taniguchi). From 1993 to 1997, served as a researcher at the Research Institute, Osaka Medical Center for Maternal and Child Health (Director Yoshinao Wada); from 1997 to 1999, served as a Postdoctoral fellow in the Division of Developmental Biology, National Institute of Dental & Craniofacial Research, the National Institutes of Health (NIH) in the United States (Director Kenneth M Yamada); from 1999 to 2002, served as an Assistant at the Institute for Protein Research, Osaka University (Professor Kiyotoshi Sekiguchi); from 2002 to 2005, served as a Lecture and an Associate Professor in the Department of Biochemistry, Graduate School of Medicine, Osaka University (Professor Naoyuki Taniguchi) and was appointed to his current position in 2006. Currently, he is conducting research on the understanding of the biological function of oligosccharides in diseases focusing on mainly cell adhesion, cancer metastasis, neurologic diseases, and lifestyle-related diseases.
The liver, which has long been known to have a very high regenerative ability, is composed of liver parenchymal cells, called “hepatocytes,” and liver nonparenchymal cells such as hepatic sinusoidal endothelial cells, hepatic stellate cells, and Kupffer cells. Liver regeneration is spatiotemporally regulated by cell-to-cell interactions and humoral factors such as cytokines and growth factors. Many of the molecules involved in cell-cell interactions and humoral factor receptors are glycoproteins.
In Greek mythology, it was said, that Prometheus begot Zeus's anger because he had given fire to humans, and was punished with the curse of ceaseless pain of his liver being eaten by a large eagle (emblem of Zeus) by day, while his liver would recover again at night, while he was kept alive. Thus, the highly regenerative capacity of the liver has long been known.
In regard to the molecular mechanism of the strong regenerative ability that only the liver has among the organs and tissues of mammals, including humans, active research is being conducted using the Higgins and Anderson model 1 in many countries. However, the manner of liver regeneration has not been confirmed until recently. It is known that it is possible to induce proliferation due to hypertrophy and division of the remaining hepatocytes after 70% partial hepatectomy using mice, and that hypertrophy and division contribute to liver regeneration to almost the same degree 2. In addition, reports have been made that support a "dispersed model," in which a few hepatocytes scattered in the liver and expressing telomerase contribute to hepatocyte regeneration 3.
Furthermore, in recent years, an activation regeneration model of hepatic progenitor cells and hepatic progenitor cells having higher proliferation ability than mature hepatocytes has drawn attention as reflecting the pathological condition of human liver disease. Elucidation of the activation control mechanism of hepatic progenitor cells is in progress, but problems remain in collection and culture methods. A report has been submitted that a cocktail of small molecules can convert rat and mouse mature hepatocytes in vitro into proliferative bipotent cells (chemically induced liver progenitors: CLiPs), and CLiPs can differentiate into both mature hepatocytes and biliary epithelial cells using hepatic differentiation stimulation 4.
Liver function is performed by hepatic parenchymal cells (hepatic cells) and sinusoidal parietal cells (sinusoidal endothelial cells, Kupffer cells, dendritic cells, natural killer (NK) cells, hepatic stellate cells, monocyte-derived macrophages). Therefore, signaling of cytokines and growth factors that induce hepatocyte proliferation, and interactions between the liver vasculature with liver regeneration and interaction with the liver vasculature-hepatocytes are important for a mechanism of liver regeneration. The ability of humoral factor signal transduction on hepatocyte proliferation has already been shown, for factors produced by nonparenchymal cells such as tumor necrosis factor (TNF) and interleukin-6 (IL-6) and hepatocyte growth factor (HGF) 5,6. Furthermore, it is reported that stimulation by humoral factor causes increased glycogen synthase kinase-3 (GSK-3) β, and GSK-3β-dependent degradation of transcription factor Snail is important for liver regeneration 7. It is also reported that liver regeneration requires an inducible angiocrine signal from the sinusoidal endothelium 8, and FGF7 is also extremely important in the process of activation of liver hepatic progenitor cells for liver regeneration 9.
Furthermore, it has also been reported that neural signals are important, such as signals transmitted from the brain to the liver by the vagus nerve, and activation of macrophages and promotion of IL-6 secretion associated with them promote liver regeneration 10.
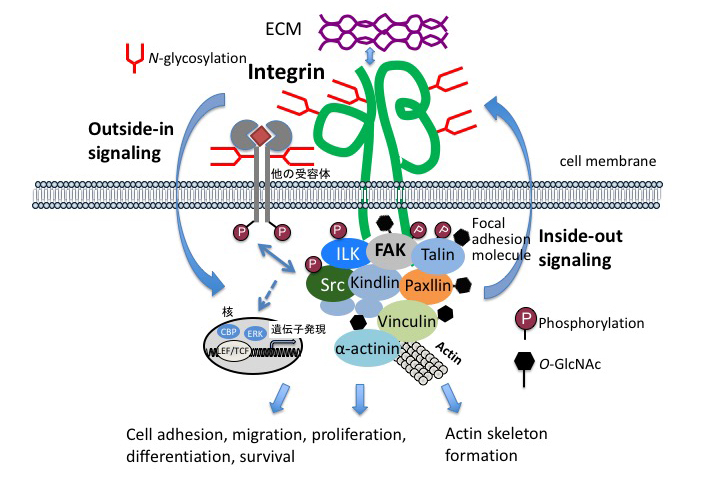
The interaction between hepatocytes and nonparenchymal cells is essential for liver construction and functional expression in liver regeneration. Hepatocytes are epithelial cells, and have the characteristic that they do not organize a clear basement membrane. In addition to cell proliferation and cell growth, the extracellular environment is also important for understanding the interactions involved in maintaining the order of cell society. The extracellular environment is provided by the extracellular matrix as a supramolecular complex of glycoproteins. The extracellular information, Cell-ECM interactions, is mediated into the cells (outside-in signaling) by cell membrane receptor integrins 11. At the origin of adhesion spots, tyrosine kinases, called “focal adhesion kinase” (FAK), mitogen-activated protein (MAP) kinases pathways that control cell proliferation, PI 3-kinase-Akt pathways that control cell survival, etc., are activated.
Integrins accumulate in focal adhesions through adhesions, and growth factor receptors also accumulate in focal adhesions and interact with adhesion molecules 12. Signals from extracellular matrix are also important in liver regeneration. Here, we focus on glycoproteins and proteoglycans, which are the main components of the extracellular matrix (ECM).
Integrins are major contributors to cell adhesion as receptors of the ECM. Interactions between cells and the ECM regulate a wide variety of cellular processes, including adhesion, migration, proliferation, differentiation, and survival. Integrins are heterodimers formed by alpha and beta chains, as shown in Figure 1. Each chain has a large extracellular domain, a transmembrane domain, and a short cytoplasmic tail. In contrast to other receptors, integrins themselves have no enzymatic activity. However, integrins bind to a large number of cytoplasmic protein kinases, such as FAK and Src (Fig. 1). Integrin activation causes these enzymatic cascade reactions, leading to changes in cell behavior and gene expression. On the other hand, a mechanism is also provided to appropriately regulate the binding to the ECM by changing the structure of the extracellular domain in accordance with the intracellular situation. In order to clarify the function of N-linked glycosylation on integrin, α5β1 integrin is used as a model molecule having 14 glycosylation sites on α chain and 12 glycosylation sites on β chain. The influence on the function of the introduced mutation in the glycosylation motif on α5β1 integrin was analyzed. N-linked glycosylations have a specific function depending on the modification site; for example, the glycosylation of β propeller domain of α5 chain is essential for cell adhesion, and the glycosylation of I-like domain at the β1 chain is essential for dimer formation and expression on the cell surface 13, and N-linked glycosylation located near the cell membrane of integrin is important for interaction with epidermal growth factor receptor (EGFR) 14. In addition, a report on X-ray crystal structure analysis has shown that the N-linked carbohydrate moiety of integrin guides the integrin binding to the extracellular matrix 15. It has also been shown that glycosylation plays an important role in integrin-mediated signal transduction, an example of which is O-GlcNAcylation, in which one molecule of N-acetylglucosamine (GlcNAc) binds to a protein present in the nucleus or cytoplasm. The O-GlcNAcylation site to be modified in the protein is the hydroxyl group of serine or threonine residue. O-GlcNAcylation can be changed as dynamically as phosphorylation: O-GlcNAc are transferred by O-GlcNAc transfer enzyme (OGT) and hydrolysis by O-GlcNAc cleavage enzyme O-GlcNAcase (OGA). O-GlcNAc has been shown to be a key regulator of cell adhesion, migration, and focal adhesion (FA) complexes 16.
In adult mice and human liver, laminin 511 (α5β1γ1), containing the laminin α5 chain, is mainly expressed in large blood vessel basement membranes such as bile duct, portal vein, hepatic artery, and central vein 17,18. However, the presence of a basement membrane is not found around hepatocytes or sinusoidal endothelial cells, and therefore, expression of laminin is not found either, but laminin 111 (α1β1γ1) is transiently expressed in the sinusoids during liver regeneration 17. In addition, in the liver of laminin α5 chain knockout mice, biliary structure formation is delayed. It is thought that morphogenesis of the bile duct is dependent on laminin α5 produced by biliary epithelial cells 19. α3-containing laminin is also specifically expressed in the bile ducts. Laminin 332 (α3β3γ2), containing α3, causes changes in cell adhesion and movement-promoting activity due to changes in glycosylation 20,21. The glycosylation on β4 chain of α6β4 integrin, which is a receptor for laminin 332, also affects the activity of laminin 332 22. Even more interestingly, core fucosylation of α3β1 integrin (core fucosylation is described later in the section on Fut8), which is the main receptor for laminin 332 and laminin 511, is essential for the function of α3β1 integrin 23. It has been suggested that changes in extracellular environment due to remodeling of glycosylation that occur during liver regeneration are important for liver regeneration.
Plasma fibronectin that has almost no core fucose structure is produced by hepatocytes 24. This is consistent with minimal expression of Fut8 in the liver 25. However, 30 years ago, fibronectin synthesized in placental tissue was reported by Endo et al. to have a core fucose structure. Increased core fucosylation has also been reported after partial hepatectomy 26. Interestingly, vitronectin also shows an increase in core fucose structure during liver regeneration.
Thus, the sugar chain changes the glycosylation according to the tissue specificity and the situation. Locally specific ECM for each tissue is important for behavior such as cell regeneration, maturation, and differentiation 27. From the approach of liver regeneration using a decellularized skeleton, it is also understood that the combination of complicated ECMs has a great influence on cells 28, acting as a sort of “tuning device” that changes the glycosylation.
Proteoglycans in which a large glycosylation (glycosaminoglycan or mucopolysaccharide) composed of a simple repeating disaccharide structure is bound to a protein interact with various cell growth factors and extracellular matrix components.
Depletion of alpha1-4-N-acetylhexosaminyltransferase (EXTL2), which functions to arrest GAG (glycosaminoglycan) elongation, increases the amount of chondroitin sulfate (CS) and heparan sulfate (HS) in liver tissue 29. An increase in the amount of glycosaminoglycan reduces HGF signal and delays the liver regeneration process 30.
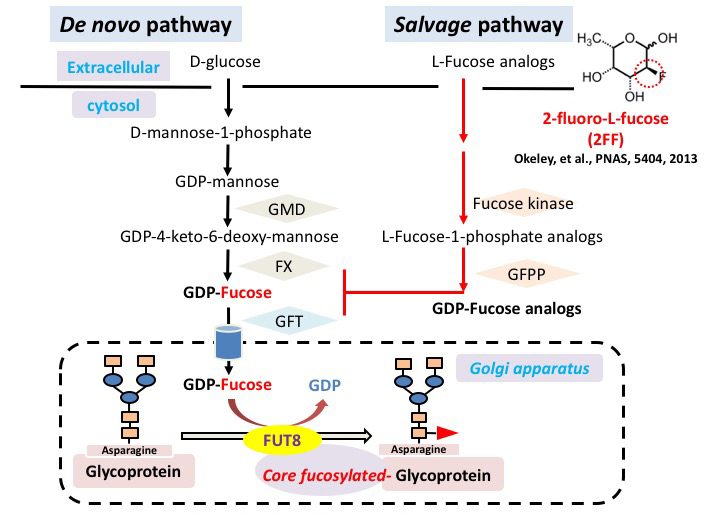
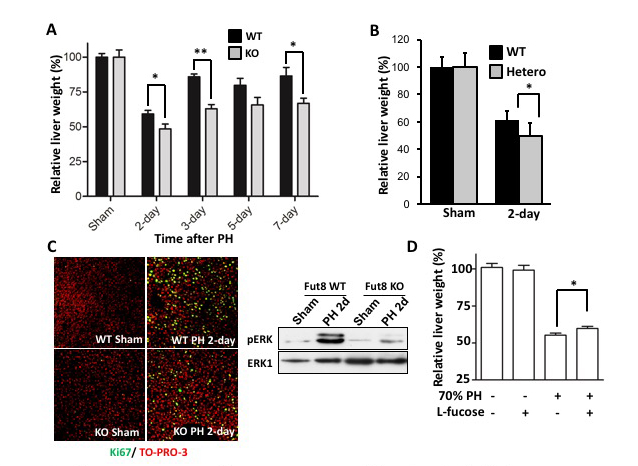
Carbohydrate moiety added to glycoproteins are considered to have various biological activities. However, few studies have shown a relationship with a specific sugar chain structure. Core fucose is biosynthesized by α1,6 fucosyltransferase (Fut8), which is attracting attention as a specific biomarker for liver cancer, as it is expressed much less in healthy liver than in other tissues. We examined the role of core fucose in the liver, focusing on its exceptionally high regenerative ability in our body tissues. The expression of Fut8 was strongly induced in the early phase (~ 4d) of liver regeneration after partial hepatectomy, and returned to the basal level in the late phase. Furthermore, Fut8-deficient mice had significantly reduced liver regeneration ability compared to wild type mice. Interestingly, not only homozygous deficient mice, but also heterozygous deficient mice in which only one chromosome is broken and the expression level of Fut8 gene is halved, were reduced in liver regeneration ability. L-fucose was orally ingested 10 days before hepatectomy to activate the salvage pathway of partially hepatectomized heterozygous mice and to increase the synthesis of GDP-fucose, a substrate for Fut8. The liver regeneration ability was then significantly restored (Figs. 2, 3) 31. This method of orally ingesting L-fucose and increasing the synthesis of GDP-fucose is also effective in CDG-IIc congenital N-linked glycosynthetic pathway disorder, due to dysfunction of the GDP-fucose transporter (ClinicalTrials.gov).
Furthermore, when liver cancer was induced using diethylnitrosamine and pentobarbital, we found that the development of liver cancer was significantly suppressed in Fut8-deficient mice. The response of Fut8-deficient HepG2 cells to EGF and HGF, which are considered to be deeply involved in the formation of liver cancer, was examined using receptor phosphorylation as an indicator. It was also found that the phosphorylation of EGF receptor and HGF receptor is reduced 32. Conversely, using 2-fluoro-L-fucose (2FF), an L-fucose analog that interferes with GDP-fucose synthesis, reduces core fucosylation levels, and downstream signals of membrane glycoproteins such as EGF receptor and integrin β1 are suppressed 33.
As far as the importance of Fut8 is concerned, Fut8-deficient patients should not be found, but three patients were reported last year. Intriguingly, in all three patients, with diverse clinical features, no abnormalities were found in the liver 34.
It is strongly suggested that core fucosylation acts as an important functional modulator in the liver and suppresses activation that disturbs homeostasis under healthy conditions, but core fucose decreases when homeostasis is disturbed, such as partial hepatectomy or canceration, and that overactivation of suppressed cells is released. In addition, Klotho mutant mice (kl/Kl), which exhibit “prosodic age,” have increased expression and activity of Fut8 in the liver with aging. On the other hand, expression of Fut8 decreases in Snell Dwarf mice (dw/dw), in which aging is delayed, and in mice that receive a calorie-restricted diet. Glycosylation in hepatocytes is significantly affected by aging 35. The body may compensate for the decrease in liver regeneration ability with aging by changing the sugar chain structure.
When a patient with liver cancer undergoes liver resection, if the function of the liver is good, up to about 60% of the liver can be resected. However, if liver regeneration is not sufficient, such as in the case of liver cirrhosis, less excision can be performed. Regardless of cancer, living donor liver transplantation is performed as the ultimate treatment for severe liver disease. In this case, regeneration of the liver is required for both the recipient and the donor. However, in the case of elderly donors, liver regeneration of donors may also involve major problems, as their regeneration ability is lower than that of young donors. Elucidation of the role of glycosylation in the regeneration of liver that is not in good condition is more important than that in liver that is in good condition.
There is no doubt that glycosylation is involved in the energetic liver regeneration mechanism. By understanding the relationship between sugar chains and liver regeneration more deeply, and by being able to extract and enhance the inherent regeneration ability of the liver through sugar chain remodeling, many patients can be salvaged. Furthermore, as one of the most attractive therapeutic strategies for liver regeneration at present, all cells used for transplantation (hepatic progenitor cells, vascular progenitor cells, and mesenchymal progenitor cells) are induced by differentiation from human iPS cells, acting as an “all-iPSC mini-liver” 36.
Although it is necessary to prepare all cell material from iPS cells, the differentiation tropism of iPS cells is also considered important. Elucidation of glycosylation involved in ECM and cell differentiation tropism (hepatic progenitor cells, vascular progenitor cells, and mesenchymal progenitor cells) of human iPS cells, and using these results for remodeling of glycosylation may greatly contribute to liver regenerative medicine.