
Asuka Morizane
Current position: Associate Professor at the Department of Clinical Application, CiRA, Kyoto University
Asuka Morizane received his Ph.D. in 2004 from Kyoto University Graduate School of Medicine. After working as a neurosurgeon in Kobe City General Hospital, he moved to Lund University as a postdoctoral researcher. He returned to Kyoto University in 2008 and assigned as an assistant professor at Kyoto University since 2012. He has held his current position since April 2019. His research theme is the application of stem cells for neurological disease. Recently he focuses on controlling immune response after cell therapy.

Jun Takahashi
Current position: Professor, Department of Clinical Application, Center for iPS Cell Research and Application, Kyoto University
Degree: Doctor of Medical Sciences
In 1986, he graduated from Kyoto University’s School of Medicine. He is a neurosurgery specialist. In 2007, he moved to the Institute for Frontier Medical Sciences, Kyoto University, as an associate professor, aiming to improve nerve regeneration by cell transplantation therapeutics. Since 2012, he has been in his current position. In 2018, he initiated a clinical trial of dopaminergic neural progenitor cell transplantation for Parkinson's disease.
A clinical trial of cell therapy for Parkinson’s disease with induced pluripotent stem cells (iPSCs) started from 2018 in Japan. Induced pluripotent stem cells have “pluripotency”, which means the ability to become many different cell types in the whole body, and also high proliferative activity. In Parkinson’s disease (PD), many dopamine neurons in the brain are lost. To compensate the lost dopamine neurons, iPSC-derived dopamine neurons are transplanted by cell therapy. To derive the dopamine neurons from iPSCs, the cells are cultured with many inducing factors. The induction technology has been developed through the intensive researches applying the knowledge from developmental biology. In the future, it is necessary to evaluate the results of clinical trials that has just started in countries around the world. Cell transplantation using iPSCs is expected to be a new treatment option for various diseases including PD.
Parkinson's disease (PD) is a neurodegenerative disorder caused by progressive loss of dopamine neurons in a part of brain called the substantia nigra, and its main symptoms are tremor, rigidity, and hypokinesia 1. In most cases, symptoms appear after 50 years of age. There are 16.3 million patients with PD in Japan, and around 600 million patients worldwide. Standard treatment of PD is medication, which is normally effective at the early stage of the disease. However, as the disease progresses gradually, the drugs become less effective and higher doses of the drugs produce side effects. Thus, additional treatment options are needed. There is a surgical option for PD treatment called deep brain stimulation (DBS). This treatment in which electrodes are implanted in the brain, is known as brain pacemaker therapy and is effective for some patients with appropriate surgical indications. Even with pharmacological treatment and DBS, the number of endogenous dopamine neurons continues to decrease. In cell therapy, new dopamine neurons are transplanted that compensate for the loss of endogenous dopamine neurons.
Cell transplantation in the central nervous system has already been in clinical use. Since the 1980s, clinical trials of transplantation of brain tissue from aborted fetuses was performed for PD and resulted in some effects in the appropriate patients 2. It was reported that some patients showed good response to the transplantation and became free of medication for a long period of time. The fetal midbrain tissue contains dopaminergic progenitor cells that will become dopamine neurons in the future. Animal studies have shown that fully mature dopamine neurons do not survive after transplantation, but immature progenitor cells do. According to previous studies, human fetuses at 11 weeks of pregnancy or earlier are optimal. In order to treat one PD patient, it is necessary to prepare midbrain tissues from 4-8 aborted fetuses. It is difficult in practice to prepare this number of aborted fetal tissues that are consistent in quality on the day of surgery. Thus, transplantation of fetal tissue is unlikely to become a standard treatment. Therefore, research has been conducted to use dopamine neural progenitors derived from stem cells such as induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs) as donor cells instead of midbrain tissue from aborted fetuses.
If iPSCs are cultured in a maintenance medium in a dish, they repeatedly divide and continue to expand as iPSCs. On the other hand, if the composition of the culture medium is changed to one favoring differentiation, the cells divide and differentiate into target cells (Fig.1a). Many signaling molecules are critical for maintenance of iPSCs. Among them, small molecules to inhibit signals of bone morphogenetic protein (BMP) and Activin are known to stimulate iPSCs efficiently to differentiate into neural cells. In addition, to induce the dopamine phenotype, fibroblast growth factor 8 (FGF8), a Wnt pathway stimulator, and Sonic hedgehog (SHH) are also added to the differentiation medium (Fig.1b). These factors have been discovered by developmental biology studies. It has been shown that iPSCs can be induced to differentiate into target neurons, mimicking the normal embryonic development of the central nervous system. The concentration, timing, and combination of factors added to the culture medium were determined in repeated experiments, and the protocol was established 3. As pluripotent stem cells have the pluripotency to differentiate into various types of cells, non-target cells appear even with the use of established protocols. Even if induction is started at the same time in a dish, differentiated cells and undifferentiated cells exist together after a certain period of time. If many donor cells are immature cells, they might become tumors or overgrowth after transplantation. On the other hand, it is difficult for too mature donor cells to survive after transplantation. An efficient technology for inducing cells toward a desired "differentiation direction" and to achieve an appropriate "degree of differentiation" is needed. For preparation of donor cells, we use a “cell sorting” technique, with antibodies used for collecting the necessary cells (Fig.1b).
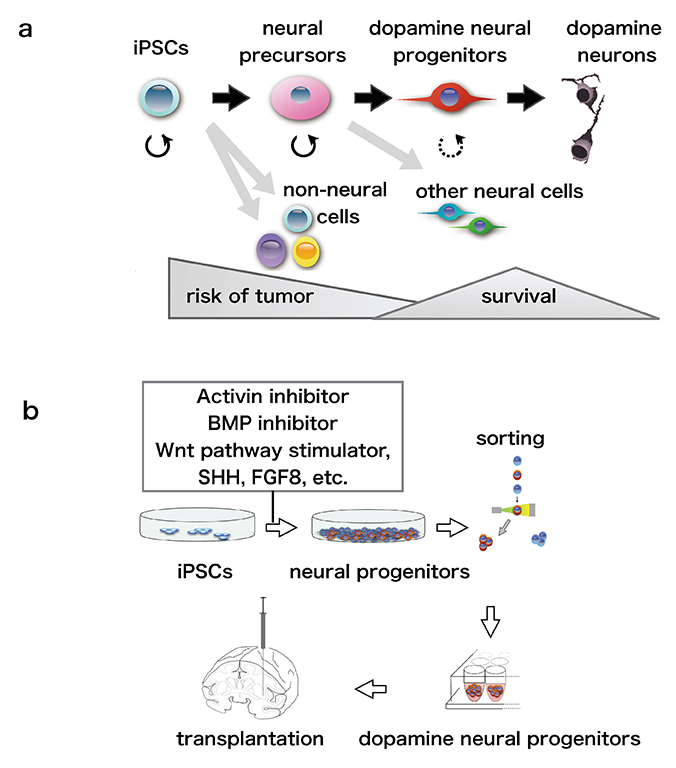
In research on PD cell therapy, it is common to use disease model animals such as mice, rats, and cynomolgus monkeys that have been depleted of dopamine neurons by injection of a neurotoxin. The iPSC-derived dopamine neural progenitor cells are transplanted into these PD model animals, and the behavioral improvement and histologically-determined cell survival are examined. In particular, the authors transplanted human iPSC-derived donor cells into a cynomolgus monkey PD model using almost the same protocol used in clinical applications. We followed the monkeys for up to two years after transplantation and confirmed the safety of the procedure and the absence of graft overgrowth or tumorigenesis by imaging studies such as magnetic resonance imaging (MRI) and histopathology. Observation using a neurological score system and video analysis showed improvements of spontaneous movement after transplantation. It was also shown that the transplanted cells were functionally active by positron emission tomography (PET) 4.
As many studies using experimental animals have confirmed its efficacy and safety, preparations for clinical application in real-world settings have started worldwide. Preparing clinically compatible donor cells at the industrial level requires more stringent conditions than at the research level. To maintain the level of product “safety” and “quality,” all aspects of production such as facilities, equipment, procedures, management, record-keeping, education, etc., the stocking of raw material, and shipment of the final product are strictly regulated by law. This includes the installation and operation of a cell processing center (CPC).
Cell therapy may not be effective for all PD patients, and it is likely that there are disease types and stages that are appropriate for cell therapy. As mentioned above, pharmacological treatment is effective in the early stages of PD. In addition, it cannot be expected that cell therapy will be effective at too advanced stages of the disease. The host neurons need to remain functional for the transplanted cells to act and exert an effect on the host.
There are three strategies for clinically applying iPSCs. The first is “autologous transplantation”, which involves establishing iPSCs from the patient's own somatic cells, inducing their differentiation into target cells, and returning them to the patient (Fig.2a) 5. This method may seem ideal because it avoids immune rejection. Practically, however, establishing and preparing iPSCs for each patient is expensive and is currently hard to do as a standard treatment. In addition, since cells generated from the patient's own cells have the same genetic background, their potential susceptibility to the disease cannot be ignored. About 10% of PD in patients is thought to be related to genetic factors. The second is stem cell transplantation from other donors (healthy volunteers; Fig.2b). In this strategy, it is possible to prepare in advance a large number of cells and perform sufficient efficacy and safety examinations. However, it is necessary to consider the immune response after transplantation. An immune response to the brain is less likely than to other organs, but there is still a chance it would occur after cell transplantation. Immunosuppressive treatments must be used for survival of the graft. The third is transplantation of special types of stem cells derived from healthy individuals, which are less likely to cause an immune response (Fig.2c). One stem cell type is human leukocyte antigen (HLA)-homo iPSC 6 HLA is a protein that is expressed on the cell surface and is involved in immune rejection after transplantation. In particular, matching the three HLA types, HLA-A, -B, and -DR, between the donor and host is important for graft survival. HLA-matched transplantation is a method in which iPSCs derived from a healthy HLA-compatible donor are transplanted to an intended recipient. If an iPSC line is established from a special HLA-homozygous donor, its HLA-haplotype will be compatible with the HLA-haplotype of many recipients. Additionally, with recent advancement of genetic engineering, some have reported the generation of iPSCs with disrupted HLA expression 7. Such iPSCs are also expected not to stimulate an immune response after transplantation.
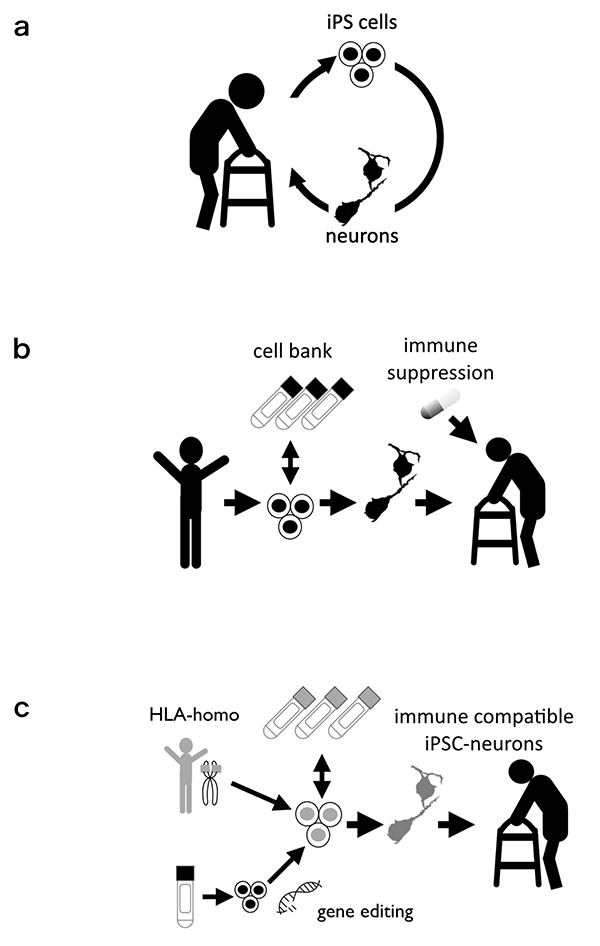
Now, in 2020, several clinical trials of cell therapy for Parkinson's disease are underway or in the planning stage; in Japan, the HLA-homo iPSC line is used, and in the US, ESCs are used 8. Like iPSCs, ESCs are pluripotent stem cells and have similar properties, but unlike iPSCs, they are established from embryonic cells, not somatic cells. The mainstay of treatment for PD will remain medical therapy for the foreseeable future. The first goal of cell therapy for PD is to become a treatment option and to benefit many patients by combining it with other treatments such as medical therapy and rehabilitation. To become a standard treatment, clinical trials of PD cell therapy should be conducted and evaluated in a concrete scientific manner with great care for safety. Researchers need to explain science and technology to the general public in an easy-to-understand manner. To establish new scientific and medical technologies, it is important to reach a social consensus on these technologies including their ethical aspects.