Cell surface glycans are tissue-specific and developmentally regulated; therefore, they are used as markers of embryonic stem cells (ESCs) such as stage-specific embryonic antigens. However, the role of glycans in stem cells has not been fully elucidated. Therefore we performed an RNA interference (RNAi) screen for glycosyltransferases essential for self-renewal and pluripotency of mouse ESCs and identified the following four glycan structures that are required to maintain the naïve pluripotent state: (1) LacdiNAc structure (GalNAc β1,4GlcNAc), (2) heparan sulfate (HS), (3) O-GlcNAc, (4) T antigen (Galβ1,3GalNAc). They regulate the leukemia inhibitory factor (LIF), bone morphogenetic protein (BMP), and/or Wnt signals that act to maintain the naïve pluripotent state and/or the fibroblast growth factor 4 (FGF4) signal that triggers differentiation. Thus, various types of glycans regulate the stem cell status; these glycan structures are evolutionarily conserved from Drosophila to mammals. In addition, ESCs and epiblast-like cells recapitulate in vitro the epiblast first cell lineage decision, allowing characterization of the molecular mechanisms underlying pluripotent state transition. Comprehensive and comparative analysis of the total glycomes of both cells revealed that overall glycosylation undergoes dramatic changes from the early stages of development. We also showed the presence of a developmentally regulated network orchestrating glycosylation changes and identified polycomb repressive complex 2 as a key component involved in this process. Here, I would like to introduce our work on stem cells, in particular, glycan functions in stem cells and the integrated control of glycan structure expression in stem cells, including attempts to apply glycans to regenerative medicine. ...and more
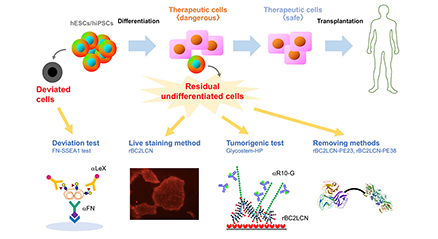
We performed comprehensive analysis of the cell surface glycomic signature of human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs). We clarified the characteristic feature of the glycome of hESCs/hiPSCs, and identified a lectin termed rBC2LCN that reacts with hESCs/hiPSCs. We then developed a technology to detect and eliminate hESCs/hiPSCs resided in cell therapy products (CTPs) using rBC2LCN. More recently, we developed a technique to detect deviated cells, cells that have deviated from the undifferentiated state of hiPSCs. Here, we would like to introduce glycan-based quality control technologies of the hESCs/hiPSCs that we’ve developed so far. ...and more