
Hakon Leffler
Hakon Leffler, received MD 1974 and PhD 1981 from Göteborg University, Sweden, 1985-86 joined group of SH Barondes at UC San Diego and moved with him to UC San Francisco 1986-1997, since then at Lund University, Sweden, now as Senior Professor. PhD and early research included isolation and structure of glycosphingolipids and their role as receptors for bacterial adhesion. From 1985 main research has been on galectins, including biochemical (discovery, specificity), structural and cellular aspects of galectins. In collaboration initiated development of potent small molecule inhibitors, some of which are now in clinical trials against fibrotic disease and cancer. For this co-founder of company Galecto Biotech AB, now Galecto Inc. (NASDAQ: GLTO).
Galectins are a family of small soluble glycan-binding proteins, implicated in a number of cellular functions and attracting attention as diagnostic and therapeutic targets for diseases such as inflammation, fibrosis and cancer. However, they occur in a bewildering number of functional contexts, intracellular and extracellular, low and high concentrations, slow and fast, low or high specificity, and there is no clear statement like: “this is the function of galectins, or even any particular galectin”. Here I will give an overview of galectins in the form of categorical statements to help raise interesting outstanding questions. The statements may not be true in all detail, but, I believe, are largely true and help make the questions clearer.
This is not a comprehensive review, and references are not selected to give fair credit to various important contributors, but rather picked as I came across them to illustrate the points made. Some questions may have already been answered and I welcome comments on this in future reviews by others.
Galectins exist in two main compartments, defined as separate because the galectins have to cross a lipid membrane to go from one to the other. One is the cytosolic-nuclear (C and N colored grey in Fig. 1) compartment, and galectins are made in the cytosol but can enter connected compartments, like the nucleus, without crossing a lipid membrane. The other main compartment is the extracellular-intravesicular (E and V colored white in Fig. 1), and galectins cannot enter it unless they first translocate across a lipid membrane. In a simplified view one can envisage a loop consisting of 1) synthesis, residence and function in the cytosol-nucleus; 2) translocation across lipid membrane induced by some signal (red unfilled arrow in Fig. 1); 3) residence and function in extracellular space and/or exocytic vesicles; 4) binding plasma membrane glycoconjugates (green in Fig. 1) and reuptake into endocytic vesicles; and 5) further population and function in the intracellular systems of vesicles, endosomes, lysosomes, trans-Golgi-network (TGN), etc. This model is well supported by experiments in its rough outline, but many key questions remain1,2. The loop is one way, because the budding (B in Fig. 1) and pinching off of a microvesicle is an energy requiring one-way process3-5. Endocytosed galectin can be recycled to the cell surface, but not by following the entry pathway backwards, instead requiring a path through a recycling endosome or TGN (R and T in Fig. 1) and re-association of a microvesicle with the plasma membrane (F in Fig. 1) in another energy requiring one-way process. Even other subloops may be one way. For example, entry into and exit from the nucleus of galectin-3, requires recognition of separate motifs within its sequence6,7.
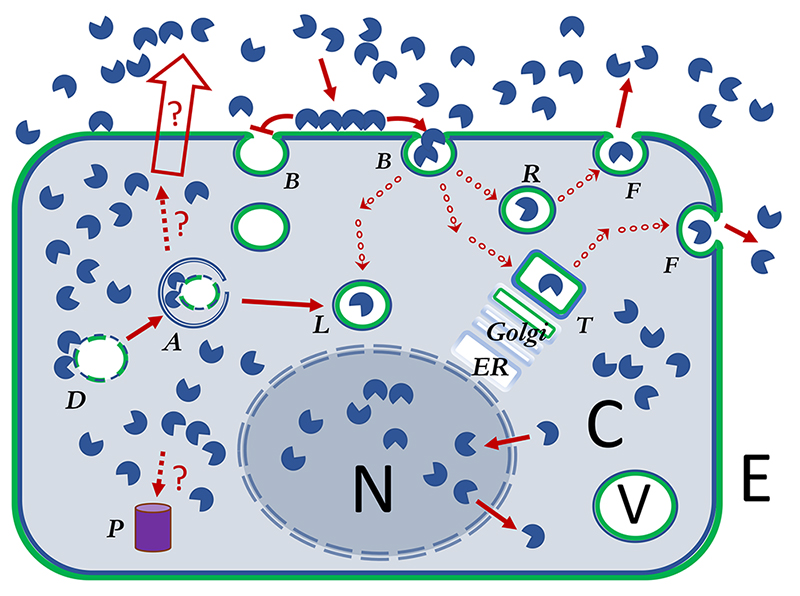
 Galectins are shown as a single CRD, but this represents all types of galectins.
Galectins are shown as a single CRD, but this represents all types of galectins. Galectins cross-linking cell surface ligands has been proposed to organize cell surface glycoprotein receptors and sometimes either inhibit their uptake by endocytosis (galectin-lattice hypothesis) or promote and drive it (GL-Lect hypothesis)
Galectins cross-linking cell surface ligands has been proposed to organize cell surface glycoprotein receptors and sometimes either inhibit their uptake by endocytosis (galectin-lattice hypothesis) or promote and drive it (GL-Lect hypothesis) Dark red arrows show pathways or impact of galectins in the cell. Uncertain but relevant pathways are shown with broken line and marked with a ?
Dark red arrows show pathways or impact of galectins in the cell. Uncertain but relevant pathways are shown with broken line and marked with a ? Arrow with a series of small circles represents transfer of galectin in ~100 nm transport vesicles, to various larger specialized vesicles (~1000 nm), such as lysosomes, TGN and various types of endosomes or exosomes.
Arrow with a series of small circles represents transfer of galectin in ~100 nm transport vesicles, to various larger specialized vesicles (~1000 nm), such as lysosomes, TGN and various types of endosomes or exosomes.
 The unfilled arrow designates non-classical secretion of galectins, by so far unclear mechanisms
The unfilled arrow designates non-classical secretion of galectins, by so far unclear mechanisms
 ) or inhibited (
) or inhibited (  ) by galectin cross-linking glycoproteins.
) by galectin cross-linking glycoproteins.Galectin function has been analyzed by a few principle types of experiments, similar as for many other molecules. One is detailed biochemical and structural studies of the galectins themselves, and this will be covered in another Glycoforum-review Footnote. Another common approach is to add a galectin to cells and see what happens. Common are also correlations between galectin expression levels and functions in cells, tissues and whole organisms, as naturally occurring or induced by some signaling moiety. A special case of this, when galectin levels are suppressed by genetic techniques in cells or KO mice, has been very informative to show when a particular galectin has rate-limiting role in particular physiological or pathophysiological functions. An interesting addition to such experiments, and unusual for other molecules, is that a function lost by genetic suppression of a galectin can sometimes be rescued by addition of galectin from outside the cell8-10. Thus, it appears as if the added galectin can repopulate the extracellular-intravesicular compartment to regain the lost function. Some consequences of this are discussed below.
A deeper understanding of galectin function comes from carefully combining these different approaches. For this, readers are urged to keep keen attention on experimental detail when reading published work. All too often in the galectin field, superficial interpretations combined with poor description of the underlying experiments have led to misleading conclusions that may divert attention from more interesting avenues of experimentation. The devil is in the details!
Some cells contain a very high concentration of galectin, e.g., galectin-3 in macrophages and epithelial cells, estimated as 5 µM in MDCK cells11, or galectin-10 as one of the most abundant proteins in eosinophils, even forming crystals after release12,13. But in other cases, a galectin has been implicated in functions, by genetic evidence such as gene ablation or suppression of mRNA, even if this galectin occurs at a much lower concentration. For example, galectin-9 in MDCK cells is expressed 100-fold less compared to galectin-314,15. In serum galectins typically occur in pM (e.g., galectin-3) or low nM concentrations. This may theoretically be functionally relevant, since only 0.3 nM of added galectin-3 or -8, respectively, was required to rescue endocytosis of cargo lost due to genetic suppression of these galectins9,10. Alternatively, it might only reflect leakage of galectin from cells or interstitial tissue, perhaps combined with degradation and inactivation, as is found for many common clinical biomarkers. The concentrations and life times in interstitial tissue, remain largely unknown for galectins as for many other proteins.
Very few studies have analyzed the half-life of a galectin, but these and indirect evidence suggest that galectins are long lived. For example, galectin-3 half-life is > 72 hours in confluent MDCK cells11. If, how and where cytosolic galectins are degraded in the cell has not been reported. Are they targeted to proteasomes (P in Fig. 1)? Ubiquitination of galectin-1 and -3 found in a screen16 would suggest this. Alternatively or in addition, the cell might get rid of galectins by release into media; the crossing of a lipid membrane from the cytosolic to the extracellular-intravesicular compartment appear to be regulated, occurring after some signals2,17,18 or under certain circumstances19,20. So, how long can a galectin stay in the cytosolic compartment, and what happens in the extracellular-intravesicular compartment? Some non-carbohydrate-recognition domain (CRD) parts of galectins are sensitive to enzymes such as metalloproteinases, elastase, and thrombin21-24, but the CRDs are still quite stable. Externalized galectins may be taken up again by endocytosis and end up in intracellular vesicles1,9,25,26 (Fig. 1). However, there is no evidence that a galectin can reenter the cytosolic compartment after exiting it. The expected ultimate end station of an externalized galectin, then, would be the lysosome (L in Fig. 1), either from secretion and reuptake by endocytosis, or from a complex process of autophagy of a galectin coated disrupted vesicle1,2,27-29 (D and A in Fig. 1). But there are no reported experiments that have addressed the question about degradation of the galectin itself in the lysosome. In a few cases where a fluorescein tagged galectin has been followed after endocytosis it appears to stay for a long time (24 hours)30. There may also be non-enzymatic inactivation or changed conformation of galectins, best known for oxidation of galectin-131,32.
In solution, the binding of galectins to purified natural ligands appears as if diffusion limited: in other words, there is nothing that hinders the fast binding once the molecules are close enough to each other. This probably also applies to galectin binding to a cell surface at 4 ºC, although it has not been studied much in detail regarding time course. At 37 ºC, a fluorescently labeled galectin added to a cell is rapidly taken up (within minutes)1,9,25,26. Endocytosed galectin first is found in microvesicles (small red circles in Fig. 1) which are rapidly transported (1 µm per second) to various larger endosomes9,26,30. There sorting takes place over times of about 10-20 minutes before new microvesicles are released to go to another type of endosome.
A fast cellular response, such as oxidative burst in neutrophils33, mobilization of intracellular calcium34, or construction of endocytic pits9 can be measured within seconds -minutes after adding galectin.
In the cytosol, the binding of galectin to a disrupted vesicle (D in Fig. 1) is another very fast process27, and the time course has been studied in detail35; galectin accumulates around the disrupted vesicles within seconds. The subsequent, coupled, process of autophagy (A in Fig. 1) also occurs fast (minutes) despite its apparent complexity. Perhaps one should expect galectin binding to other cytosolic ligands also to be very fast, but analysis of this has not been reported.
In contrast, most experiments on galectins have time scales much slower than the above-mentioned processes; thus, there is a need for more analysis of the short time scales.
In most experiments when a galectin is added to cells, the studied effect is measured after hours – days. Since, at a molecular level, galectins act fast, one has to ask: what is it that takes time? The late measured effects may be the result of many molecular steps, linear, branched or in more complex network form, some perhaps directly involving galectins but others not. Alternatively some molecules need to be slowly built up or degraded. In any case it remains unclear what the actual direct role of galectin was, and this needs to be dissected further. In experiments where expression of a galectin is disrupted by genetic technology, in cells or in animals, the time scale is again, by necessity, long before a readout is possible. So, the measured effect may be due the loss of the galectin, but also to any number of other indirect cellular responses to it. Nevertheless, such experiments show that the disrupted galectin was in some sense rate limiting for the measured process, directly or indirectly.
The development of potent specific small molecule galectin inhibitors will permit analysis of shorter time spans between galectin inhibition and measured effect, but this interesting possibility has so far only been explored to a limited extent36.
In solution or on an artificial surface a galectin has an apparent equilibrium affinity for natural-like ligands, such as glycoproteins, of Kd in the range 0.1 - 5 µM. For galectins, multivalency is important to cross-link ligands, but it does not enhance affinity or avidity much37-39. Moreover, in many cases galectins are monovalent at the concentration they have an effect. For example, galectin-1 is almost only monomeric at concentrations below 0.1 µM 38, and galectin-3 below 100 µM 37. They appear to form dimers or multimers upon ligand encounter as an essential step in their biological function, but the details of timing, cross-linking and affinity effects of this remain largely unknownFootnote.
Saturation binding to cells is hard to achieve, because there galectins bind many different ligands with a range of affinities, but from rough half maximum concentrations required for binding or biological effects, and some more detailed studies, the affinity for cell surface ligands also appear to be in the range 0.1 - 5 µM 23,40-43, and this also agrees with the concentration of added galectin for some fast signaling effects, for example oxidative burst in neutrophils44.
However, there are some striking exceptions. As mentioned above, when expression of a galectin was disrupted, the lost effect could sometimes be rescued by adding galectin protein from the outside. In some such experiments (e.g., on endocytosis), incubation with only sub-low nM galectin concentrations was required for the rescue, and in fact incubation with higher concentrations inhibited the rescue effect9,10. How is this possible? Has some natural ligand with much higher affinity been overlooked so far? The development of potent synthetic galectin inhibitors show that low nM monovalent affinity of the galectin carbohydrate-binding site is possible45,46, although not yet found among natural ligands. Is there a particular case of sterically precise multivalency that leads to higher affinity in these cases? Or is the reversible galectin-binding coupled to an immediate subsequent irreversible step like endocytosis, giving a different dose-response compared to the usual expected from an equilibrium binding reaction?
At the other end of the spectrum are a number of studies of galectin induced apoptosis. There cells are incubated with very high concentrations of galectins, such as 10-20 µM for galectin-1 and 5-10 µM for galectin-3, for many hours34,47,48. Most studies have assumed that one or more specific receptors are activated to induce the apoptosis and had the goal to identify such receptors. Now, reinterpretation of the data is needed, taking into account the long-time delay, different endocytic pathways, and other factors. In one line of thought, for example, a cell incubated with 20 µM galectin for typically 8 hours at 37 ºC, might have taken up much more galectin than it can handle, and the stress and/or injury from this overload might be the reason for the apoptosis and/or PS exposure. As mentioned above, little is known about the long-term fate of endocytosed galectin, where it is degraded, if it is made to leave the cell again, and if there is a limit on how much a cell can take up. In some studies galectin induced PS-exposure is reversible after galectin in the medium has been removed, which perhaps is due to the cell getting rid of its overload of galectin.
The formation of a cross-linked lattice between galectins and cell surface glycoprotein receptors (indicated by aggregated surface galectins in Fig. 1) has been a fruitful model to explain galectin inhibition of endocytosis of some receptors and their availability for signaling49,50, and how this might be coupled with the degree of branching of N-glycans, including the effect of and metabolic availability of GlcNAc51. Most studies of this, as depicted in recent reviews, have focused on the lattice at the cell surface and endocytosis of the affected glycoprotein receptors, but not on the endocytosis of the galectin itself52. Other studies have found, as mentioned above, that galectins added to cells tend to be rapidly endocytosed, and may increase endocytosis of selected glycoproteins, mainly through clathrin independent endocytosis; for galectin-3 a detailed mechanism has been proposed termed the GlycoLipid-Lectin (GL-Lect) hypothesis9,26, There is not much evidence for a resident galectin lattice at the cell surface, although this might vary between cell types. One elegant careful study has visualized galectin-3 aggregates on cells by FRET53, and tried to estimate their stability , although galectin at the cell surface or early after endocytosis would have been hard to distinguish. In neutrophils the aggregates lasted for about 10 minutes (unprimed cells) or less (primed cells). In endothelia cells, aggregates were seen at cell junctions and appeared more stable as they were not repopulated by endogenous galectin 30 minutes after photo-bleaching. Thus, in most cases galectin interactions are most likely rapidly turned over, perhaps breaking down and reforming cross-linked lattice areas. In this loosened view, cell surface galectins or temporary galectin lattices can either inhibit or enhance endocytosis of different receptors50. Moreover, as mentioned above, endocytosis is not a reversible equilibrium but a one-way process. For a galectin to come out to the surface again, it must go through a recycling pathway composed of distinct endosomal compartments each providing different local environment (e.g., pH) that might affect its sorting2,54. This was illustrated for example in a study where labelled galectin-8 was added to human fibroblast like cells (HFL-1, Carlsson, M. and Leffler, H., unpublished). The galectin was found in intracellular vesicles after a few minutes, but after about 10 minutes it started accumulating in a clear plasma membrane close location. This was due to recycling because in a recycling deficient cell from Nieman-Pick-C disease, the galectin-8 remained in intracellular vesicles, and did not accumulate along the membrane. Results like this open the questions, whether the recycled galectin enters a different pool at the cell surface, compared to the galectin added from the outside or already there, and how fast the two pools interact and mix.
Galectins have in common affinity for galactose with a defining binding site1. For galectins-1 and -3, the disaccharide Gaβ1-4GlcNAc (LacNAc) is the simplest preferred moiety within natural glycans, but other galectins may prefer other Galβ-containing core disaccharides43,55. Moreover, additional saccharides or other moieties on either side of the core disaccharide, can enhance or block the binding, resulting in the well-known fine specificity that differs between different galectins42,43,55,56 Footnote. The biological roles, however, of such fine specificities have been explored only to a limited extent. Here are some examples.
One source of fine specificity comes from what the core disaccharide is linked to, e.g. in which N-glycan is it found. Cell surface and other cellular glycoproteins contain a range of N-glycans, and among them the hybrid and complex type, containing galactose are major binding sites for galectins based on comparing wt cells with those where different glycosylation enzymes have been genetically ablated41,57,58. These can typically have one – four antennae, each containing a potentially galectin-binding LacNAc-residue, but different galectins have different selectivity among the N-glycans41,59,60, and structures beyond the LacNAc-residues appear to be important, possibly including also protein parts of a glycoprotein Footnote. Low or sub µM affinity of a natural soluble glycoprotein for galectin-3 requires the presence of at least one triantennary N-glycan8. The intact glycoprotein binds much better than the free glycan, indicating that the binding also involves interaction between the galectin and parts of the protein. Even a glycoprotein like transferrin, with only two N-glycans, can be fractionated by affinity chromatography into a galectin-3-binding pool with mainly tri-antennary N-glycans and a non-binding pool with only biantennary N-glycans8. Similarly, haptoglobin with 4 N-glycans can be fractionated into a galectin-1 binding pool and a non-binding pool having or lacking tri- tetra-antennary N-glycans, respectively61. These two serum glycoproteins deliver iron or hemoglobin, respectively, to tissue cells by receptor mediated endocytosis. In each case the galectin-binding pool follows a different intracellular pathway after endocytosis, compared to the non-binding pools, while both pools bind equally to the cognate surface receptor. In other examples galectin-3 binds fibronectin from tissue but not plasma59, and galectin-1 but not galectin-3 binds CD4 and have different effects on HIV-infection62.
These examples suggest that also a particular cell surface glycoprotein may consist of galectin-binding and non-binding pools that may have different fates within the cell. In a complex glycoprotein with many N-glycosylation sites, like α5β1 integrin63, the N-glycans are not evenly distributed, and potential galectin-binding triantennary glycans concentrated to certain places, making the question of fine specificity even more complex.
Another source of fine specificity and selectivity is the linkage of different saccharide moieties at the non-reducing side of the core disaccharide, mainly to the 3-position of the Galβ-residue. For example, a recycling intracellular path of galectin-8 after endocytosis, requires expression of sialic acid by the cell, and the preference for α2-3 sialylated galactose of its N-terminal CRD, unique among vertebrate galectins43,64,65. If this expression and affinity, respectively, are lost by mutation, galectin-8 will be sorted towards lysosomes64. In other studies, the preference of the N-terminal Gal-8 CRD for sialylated galactose directed this galectin to a selected set of disrupted intracellular vesicles, different from those attracting e.g., galectin-366. A special case is the apparent bactericidal effect of galectins on bacteria expressing blood-group A and B-determinants67. Such determinates consist of a Galβ-residue with added Galα or GalNAcα to 3-position and Fucα to the 2-positions; this may enhance binding to some galectins and decrease it for others42,44,56, but the biological roles, if any, of this within eukaryotic cells remain unclear.
These, and other examples show that galectin fine specificity can clearly distinguish subsets of glycoconjugate ligands in the cell and have functional consequences44. Thus, there is an intricate play of fine galectin selectivities the functions of which largely remain unexplored.
Some studies argue that galectin binds mainly LacNAc-residues with low affinity, and that it is the density of such residues that determines degree of binding; as a consequence different glycans but with similar numbers of LacNAc can be bioequivalent52. However, the examples above, show that fine specificity among glycans even with same number of LacNAc-residues also plays key roles for functions. If a certain glycan extension is lost, another may compensate for certain functional effects, but probably not all. Even a galectin KO mouse is largely healthy in animal house conditions, showing that nature has multiple ways of compensating and solving the necessary functions.
Professor Kasai has made the interesting remark that low affinity interactions can still lead to high resolution selectivity in a chromatography like setting, where equilibria reform multiple times during flow68. May be some steps during the galectin-loops described above can have some of these features, for example inside sorting endosomes or on the cell surface.
Galectins may also form fuzzy but selective interactions, perhaps in liquid droplet like complexes, both via recognition of carbohydrate and non-carbohydrate ligands37,69,70, or at high (albeit unnatural) concentrations without ligand71,72. This will be Discussed in a separate GlycoforumFootnote.
Some ingredients: synthesis and residence at relatively high concentration in cytosol, externalization induced by some signal, recycling into endosomes and population of the intracellular vesicular network (Fig. 1). Along the way, interaction with various ligands, fast like on disrupted vesicles or slow, and excursions into loops like into nucleus and back. All the many interactions are going on at the same time in a cell. How do we view function of a particular galectin based on that? Is it a homeostatic regulator, or is it a brigade ready to go when injury or enemy attack happens? Or a combination? If a regulator, what is regulated? Integrity of intracellular vesicles is a good candidate, by repair or removal after damage2,29,73,74. Cell surface residence time and/or intracellular targeting of certain glycoprotein glycoforms are other good candidates well supported by experiments9,10,49,50,52. Driving and/or monitoring of plasma membrane size and turnover? Selective sorting of particular incoming glycoprotein glycoforms8. Can one define a kind of galectin tonus or set point reflecting these functions?
Most evidence indicates that galectins function in vivo within the cells that made them or after transfer to cells nearby (paracrine action). The concentrations in serum appear too low for galectins to act over long distance, but as mentioned above, some effects required very low galectin concentrations, so nothing can be ruled out. Multiple in vivo experiments using e.g., adoptive transfer of bone marrow or site- specific gene deletion technology75-77, show that galectin made in one cell can affect another. This combined with the fact, described above, that externally added galectin can rescue functions lost due to genetic deletion of galectin, make it very plausible that galectin from one cell can be delivered to and tune another cell. On the receiving cell, galectin can e.g. drive the endocytosis of glycoproteins according to the GL-Lect mechanism9,26, and populate intracellular vesicles in the endocytic and exocytic pathways, perhaps affecting sorting of glycoproteins, altering surface exposure of receptors49,50,52 or cell handling of incoming glycoproteins like transferrin or haptoglobin8,61. Both in fibrotic disease and cancer, for example, galectin-3 delivered by macrophages appears to be a key component to regulate fibroblasts in fibrosis or protect cancer cells75,76. But so far, the research community has only scratched the surface. There are so many more additional interactions, pathways and biological effects for further study. Is there an in vivo galectin tonus? Why is expression of galectin-3 induced in conjunction with fibrotic disease, and yet, as based on animal experiments, appear to make the disease worse? What is the good beneficial side of the increased galectin-3 expression? As opposed to animal experiments, fibrosis in humans and many other chronic diseases develop over long time. Shall one view them as a continuous deterioration in some regulation, or as a series of small and rare injuries. What role does galectins play in either of these scenarios?
It has been possible to design potent specific small molecule inhibitors of the galectin carbohydrate binding site – galectins are druggable45,46. Some of these have shown promising therapeutic effects in animal models71,72, and some are in clinical trials with promising effects on biomarkers for disease78, while therapeutic effects need to await finish of larger studies. One interesting finding both in animals and humans is that galectin-3 inhibitors appear to decrease the level of galectin-3 in e.g., macrophages75,76,79. What is the mechanism for this? As for most therapeutic drugs except antibiotics, one shall not expect galectin inhibitors to completely wipe out galectin function, but rather to inhibit partially and perhaps change a galectin regulatory set point.
There is a large number of papers describing effects of modified citrus pectins and other plant polysaccharides, claiming that the effects are due to galectin inhibition. This claim is wrong. These polysaccharides bind galectins poorly and are not at all specific, neither among galectins nor compared to other molecules80,81. So, their effects should be interpreted without the presumption that they function as galectin inhibitors, as the presumption is not well founded in experiments. This is another example of “the devil is in the details” mentioned previously.
I thank the following for carefully reading the manuscript and providing comments: Ludger Johannes, Inst. Curie, Paris, Ralf Jacob, University of Marburg, Germany, Ulf J. Nilsson, Lund University, Sweden, and Rob Slack, Galecto Inc., Stevenage Bioscience Catalyst, UK. Disclosure. Hakon Leffler is a co-founder, shareholder and consultant with Galecto Inc., a company developing small molecule galectin inhibitors for clinical use.