
Keita Nishiyama
Department of Microbiology and Immunology, Keio University School of Medicine
- Brief curriculum vitae
2012, Master in Agriculture, Graduate School of Veterinary Medicine and Animal Sciences, Kitasato
University.
2015, PhD in Agriculture, Graduate School of Veterinary Sciences, Kitasato University.
2015-2019, Assistant Professor, Laboratory of Microbiology, School of Pharmacy, Kitasato University.
2019-present, Lecturer, Department of Microbiology and Immunology, Keio University School of
Medicine.
I am interested in the molecular mechanisms underlying bacterial colonization of the gastrointestinal tract. In particular, my focus is on the mechanism of bacterial adhesion to mucus.

Takao Mukai
Professor, Department of Animal Science, School of Veterinary Medicine, Kitasato University
- Brief curriculum vitae
Bachelor of Science in Agriculture, Tohoku University, in 1985. Master of Science in Agriculture in 1987. I received a PhD degree in 1990 from the Graduate School of Agriculture, Tohoku University. After that, I was appointed to the Department of Animal Science, School of Veterinary Medicine, Kitasato University, where I worked as an assistant professor, lecturer, and associate professor before assuming my current position.
My research theme in graduate school was structural analysis of polysaccharides produced by lactic acid bacteria, which led to my interest in glycoscience from a microbiological perspective. Since then, I have been conducting research on the interaction between intestinal bacteria and glycoconjugates.
Sialic acid was first isolated from bovine submaxillary gland mucin by Blix in 19361, and more than 50 types of sialic acid have been identified to date. Sialic acid is a general term for neuraminic acid derivatives of negatively charged carboxyl group-containing nine-carbon acidic amino saccharides, which can be classified according to the carbon-5 position into three types: N-acetylneuraminic acid (Neu5Ac), N-glycolylneuraminic acid (Neu5Gc), and deaminoneuraminic acid. Sialic acid is closely related to various biological phenomena and is essential for maintaining the development of the human brain and nervous system and regulating the immune system2-4. Sialic acid is also associated with colonization by symbiotic microorganisms, including bacteria, viruses, and fungi5. Therefore, sialic acid is extremely important for understanding the host-microorganism symbiotic relationship. In this study, we review sialic acid metabolism by the gut microbiome and its effects on the physiological state of the host.
Sialic acid is distributed in many parts of the body as a component of cell surface glycoconjugates6. Sialic acid is abundant in human breast milk. In the early stages of lactation, the amount of sialic acid can be as high as 1.55 g ± 0.64 g/L, and it gradually decreases with the lactation period7. More than 70% of sialic acid (Neu5Ac) in human breast milk is bound to milk oligosaccharides, mainly 6'-sialyllactose (6'-SL, Neu5Acα[2-6]Galβ[1-4]Glc) or 3'-sialyllactose (3'-SL, Neu5Acα[2-3]Galβ[1-4]Glc). Human milk also contains abundant glycoproteins and glycolipids, including lactoferrin, secretory immunoglobulin A, and gangliosides, whose glycan moieties can be partially sialylated8,9. In the body, sialic acid rarely exists as a single substance.
Sialic acid is also abundant in mucin O-glycans, and the non-reducing terminus of the type 3 core structure of secretory MUC2 type mucin in the colon is frequently modified by sialic acid10. However, sialic acid modification is greatly affected by the host’s existing health conditions, such as inflammatory bowel disease11.
Commensal and pathogenic bacteria have developed the ability to utilize sialic acid as a carbon source. The enzymes involved in sialic acid metabolism form a Nan cluster that is widely conserved in bacteria12. Typically, sialic acid is internalized by the transporter NanT; then, intracellular sialic acid is continuously metabolized by five enzymes, N-acetylneuraminate lyase (NanA), N-acetylmannosamine-6-P epimerase (NanE), N-acetylmannosamine kinase (NanK), N-acetylglucosamine-6-phosphate deacetylase (NagA), and glucosamine-6-phosphate deaminase (NagB), and finally converted to fructose-6-phosphate, which is used as a substrate for glycolysis12. Recently, several types of transporters involved in sialic acid uptake have been identified, and single strains of some bacteria possess several kinds of sialic acid transporter13. A search in 1,902 bacterial genome sequences deposited in 2009 revealed Nan clusters in 46 species, 42 of which were human-associated species14. Of note, these 42 bacterial species included pathogenic (or opportunistic) bacteria, such as Clostridium perfringens, Escherichia coli, Salmonella enterica, Vibrio cholerae, and Yersinia enterocolitica.
Although the Nan pathway is widely distributed in intestinal bacteria, bacteria that utilize sialo-oligosaccharides are not predominant. We examined the accumulation of Neu5Ac and 6'-SL in 10 bacterial strains belonging to Bacteroides, Bifidobacterium, and Clostridium, which are predominant genera in the human intestine (Figure 1). Six bacterial strains (Bacteroides fragilis, Bacteroides dorei, Bifidobacterium breve, Clostridium scindens, Clostridioides difficile, and C. perfringens) were able to grow in the medium supplemented with Neu5Ac. On the other hand, four strains, B. fragilis, B. dorei, Bifidobacterium bifidum, and C. perfringens, grew in medium supplemented with 6'-SL. These results indicate that bacteria, such as B. breve and C. difficile, which can metabolize sialic acid (Nan pathway), are unable to consistently utilize sialo-oligosaccharides. Thus, what strategies are these bacteria using to utilize sialic acids in the gut? These bacteria require nutritional symbiosis with sialidase-positive bacteria to obtain free-form sialic acid.
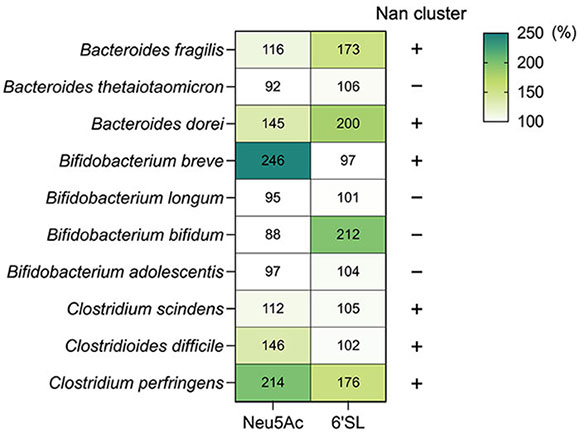 Figure 1. Sialo-oligosaccharide metabolism by intestinal bacteriaNeu5Ac and 6'-SL (Neu5Acα[2-6]Galβ[1-4]Glc) metabolism by intestinal bacteria. Each bacterium was cultured in semi-fluid Gifu Anaerobic Medium for 24 h. The OD600 values of the medium without sugar are indicated as 100%. Bacterial growth percentage after Neu5Ac or 6'-SL supplementation is shown in the heat map (each number represents %). The presence or absence of the Nan pathway in each bacterium is indicated by + and – [based on the authors’ original data and Ravcheev and Thiele, 201715].
Figure 1. Sialo-oligosaccharide metabolism by intestinal bacteriaNeu5Ac and 6'-SL (Neu5Acα[2-6]Galβ[1-4]Glc) metabolism by intestinal bacteria. Each bacterium was cultured in semi-fluid Gifu Anaerobic Medium for 24 h. The OD600 values of the medium without sugar are indicated as 100%. Bacterial growth percentage after Neu5Ac or 6'-SL supplementation is shown in the heat map (each number represents %). The presence or absence of the Nan pathway in each bacterium is indicated by + and – [based on the authors’ original data and Ravcheev and Thiele, 201715].
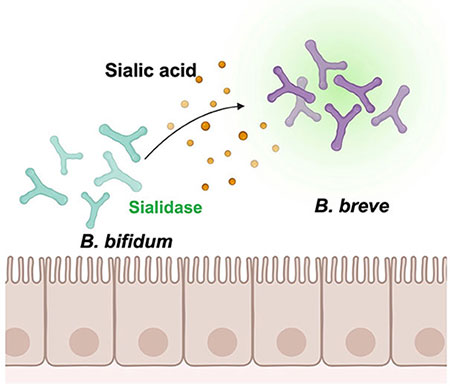
Sialic acid cross-feeding by intestinal bacteria refers to nutritional symbiosis between heterologous bacteria. In this context, sialic acids bound to milk oligosaccharides and mucin glycans are released by bacteria possessing sialidase; subsequently, free sialic acids are consumed by bacteria possessing the Nan pathway. Based on a delineation of the cross-feeding patterns between Bifidobacterium species, we obtained an overview of sialic acid-mediated cross-feeding of intestinal bacteria. As shown in Figure 1, B. breve can utilize Neu5Ac, but not 6'-SL. In contrast, B. bifidum lacks the Nan pathway and possesses an extracellular sialidase. When B. breve and B. bifidum were co-cultured in a medium containing 6'-SL, B. breve exhibited rapid growth; in contrast, B. breve did not grow in co-culture with a strain of B. bifidum containing a mutant sialidase gene (siaBb2)16. This indicated that B. breve depends on B. bifidum sialidase to obtain free sialic acid (Figure 2). In fact, the four strains (B. fragilis, B. dorei, B. bifidum, and C. perfringens) carried sialidase homologs and grew on 6'-SL-supplemented medium15.
In non-human mammals, a symbiotic relationship mediated by milk oligosaccharides has been reported in the intestinal microbiome of infant rats. The major oligosaccharides in rat milk are 3'-SL and 6'-SL, with 3'-SL being 10 times more abundant than 6'-SL. On day 5 of lactation, the 3'-SL concentration reaches approximately 40% of the sugar content in rat milk17. Therefore, it has been suggested that sialidase-expressing bacteria are present in the infant rat intestine. Recently, Enterococcus gallinarum was identified as a sialidase-expressing bacterium in the infant rat intestine18. Notably, E. gallinarum utilizes free sialic acid for its growth and provides lactose to another species, Lactobacillus19. The authors suggested the importance of cross-feeding during the development of the Lactobacillaceae-dominant infant rat microbiome18,19.
Furthermore, experiments using germ-free animals have shown that sialidase-expressing bacteria affect the amount of sialic acid in the intestine. The Bacteroides genus has an excellent polysaccharide metabolism mechanism named the polysaccharide utilization locus (PUL), which consists of genes encoding multiple carbohydrate-degrading enzymes and transporters20,21. Notably, when administered to germ-free mice, B. fragilis hardly increased the concentration of free-form sialic acid, while Bacteroides thetaiotaomicron increased the concentration of free sialic acid derived from mucin22. The reason for these results is that B. fragilis possesses sialidase and Nan pathways and can utilize free sialic acid, whereas B. thetaiotaomicron lacks the Nan pathway and cannot metabolize free sialic acid. Furthermore, when Salmonella Typhimurium or C. difficile were co-administered to mice with B. thetaiotaomicron, gene expression of the Nan pathway was altered in S. Typhimurium and C. difficile22. This indicates that consumable free sialic acid produced by sialidase-expressing bacteria can alter the glycolytic metabolism profile of heterologous bacteria.
Sialo-oligosaccharides metabolized by microorganisms through cross-feeding promote the growth of intestinal bacteria and alter their metabolism. In particular, the metabolites produced exert wide-ranging effects on the physiological state of the host.
In a cohort study of Malawian infants, the breast milk fed to malnourished children contained low amounts of sialo-oligosaccharide and fucosyl-oligosaccharide23. The microbiota from malnourished infants was transferred to germ-free animals, and the animals were subsequently fed sialo-bovine milk oligosaccharide (S-BMO), which contains high amounts of 3'-SL and 6'-SL derived from cheese whey, along with the Malawian diet. The results showed that S-BMO administration caused changes in the levels of branched-chain amino acids and various fatty acids and consequently promoted bone formation and weight gain in the animals23. Notably, these changes were not observed in the germ-free animals treated with S-BMO. Subsequently, researchers focused on intestinal bacteria. They found that the microbiome composition hardly changed, whereas PUL gene expression in B. fragilis was remarkably altered. Furthermore, in vitro culture experiments showed cross-feeding between B. fragilis, a S-BMO-degrading bacterium, and E. coli, a sialic acid-utilizing bacterium, via sialic acid derived from S-BMO23. Therefore, S-BMO cross-feeding promoted diversification and formation of the intestinal microbiome, which in turn promoted host growth. These researchers also clarified the underlying mechanism via in vivo experiments; they transplanted bacteria isolated from malnourished infants into germ-free mice and treated the mice with S-BMO24. They found that S-BMO administration increased succinate concentration in the intestine due to succinate production by intestinal bacteria. In addition, increased succinate levels in the intestine activated cell signaling pathways related to immune responses via Th2 cells24.
Interestingly, it has been shown that cultivation of human fecal samples in broth supplemented with SL increased the number of Bacteroides spp. and altered the amount of short-chain fatty acids, such as propionate and butyrate25. Recently, carbohydrate metabolism was also shown to be relevant to Bacteroides resistance to butyrate26. Butyrate inhibits B. fragilis growth, whereas butyrate tolerance of B. fragilis is affected by the acyl-CoA transferase activity of each strain, as well as by the metabolism of individual carbohydrate components26. For example, when B. fragilis metabolizes 3'-SL or lacto-N-neotetraose, it exhibits a higher tolerance to butyrate than 2'-fucosyllactose26. Thus, sialo-oligosaccharide metabolism by intestinal bacteria has a remarkable effect on colonization and metabolic state, and the resulting changes in bacteria-produced metabolites may be a key factor regulating homeostasis in the host intestinal environment.
Neu5Ac and Neu5Gc are major sialic acids in mammals. However, humans exhibit a species-specific deficiency of Neu5Gc due to pseudogenization of the cytidine monophosphate Neu5Ac hydroxylase gene27. The only difference between Neu5Ac and Neu5Gc is the hydroxy group in the side chain, which has various effects on the physiological activity of the host. For example, Neu5Gc content in milk is approximately 3% of the total sialic acid, while red meat, such as beef and mutton, is rich in Neu5Gc (19-43%)28. In some cases, after consuming foods containing Neu5Gc, the human body produces anti-Neu5Gc antibodies, which are related to the development of chronic inflammation. According to experiments in Neu5Gc-deficient mice, administration of Neu5Gc or injection of anti-Neu5Gc antibodies in mice induces inflammation and tumorigenesis in the liver29. Therefore, Neu5Ac is a naturally occurring substrate in humans, whereas Neu5Gc may be an antigenic agent that induces inflammation.
Neu5Gc has also been reported to exert a significant impact on gut microbiome composition. When mice were fed porcine submaxillary gland mucin rich in Neu5Gc or swallow’s nest rich in Neu5Ac, Neu5Gc-rich food-fed mice showed an increase in Bacteroidales and Clostridiales30. Indeed, several bacterial sialidases have shown stronger activity against Neu5Gc than against Neu5Ac. The authors mentioned that, in the human intestine, sialidase-expressing bacteria cleaved Neu5Gc derived from food, and bacteria that possess the Nan pathway metabolize Neu5Gc by cross-feeding, which may reduce anti-Neu5Gc antibodies and contribute to the suppression of inflammation30. Neu5Ac metabolized by the bacterial Nan pathway produces acetic acid as a by-product, whereas Neu5Gc is converted to glycolic acid12. These differences in byproducts may lead to unexpected changes in host physiology, and further research is needed to understand both the microbiome and host physiology.
Cross-feeding of sialic acids by intestinal bacteria is a survival strategy acquired through the coevolution of hosts and microorganisms in the intestinal environment. In addition, sialic acid metabolism by intestinal bacteria is a process of nutrient acquisition, as well as a signal-like action that generates various changes in metabolic pathways. Recent experiments have also shown that sialo-oligosaccharide metabolism by intestinal bacteria significantly affects the physiological state of the host. Further studies are needed to determine the dynamic effects of sialo-oligosaccharides on the intestinal microbiome and host.
This content partially overlaps with the content of an article by the authors published in the Dairy Science Symposium 2021 Short Review of Milk Science, and Journal of the Japanese Dairy Science Association.