
Hiroaki Kitagishi
Department of Molecular Chemistry and Biochemistry, Faculty of Science and Engineering, Doshisha University. Professor
Hiroaki Kitagishi received his PhD in 2006 from Doshisha University under the supervision of Professor Koji Kano. After two years of heme protein engineering studies with Professor Takashi Hayashi at Osaka University as a postdoctoral fellow, he was appointed assistant professor at Doshisha University in 2008. From 2009 to 2010 he investigated virus-like nanoparticles with Professor M. G. Finn at the Scripps Research Institute as a visiting researcher. He was promoted to associate professor and subsequently to full professor at Doshisha University in 2014 and 2020, respectively.
Cyclodextrins (CDs), cyclic oligosaccharides, are water-soluble host molecules that can include various kinds of guest molecules utilized not only in basic research but also as food additives. CDs with oligo-glucose structure have interesting properties; however, CDs can be chemically modified and thereby acquire new functions. We are studying the artificial hemoglobin “hemoCD”, which consists of a per-O-methylated CD dimer and iron porphyrin (FeTPPS). In this article, I describe the strategy of hemoCD preparation and its medical applications.
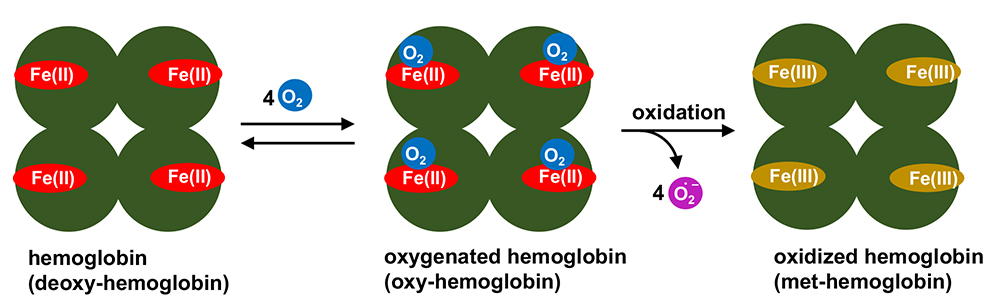
In our adult bodies, we have approximately 4-5 L of blood. Blood contains various components: one component, hemoglobin, has a role in oxygen transportation. Red blood cells have no nuclear structure and a high concentration of hemoglobin. A cofactor called heme is included in each of the four subunits of hemoglobin (Figure 1). Heme usually keeps its iron(II) oxidation state and reversibly binds oxygen (O2) depending upon the partial pressure of O2 in the atmosphere. The important function of hemoglobin is to pick up O2 in the lung where the O2 partial pressure is relatively high, and release it in the O2-deficient terminal tissues. Notably, heme in iron(II) state is unstable and easily oxidized to its iron(III) oxidation state. The oxidized iron(III) heme cannot bind O2 and thus it is regarded as biologically inactivated hemoglobin (met-hemoglobin). Iron(II) heme is protected from oxidation (called autoxidation) by a hydrophobic heme pocket in globin protein1.
Since the 1970s, there were many studies of compounds mimicking the chemistry of hemoglobin’s O2 binding function. The basic design of these studies was based on synthetic porphyrin iron complexes. The point of creating an O2-complex in the porphyrin is to protect the porphyrin iron(II) complex from autoxidation. Prof. J. P. Collman and his co-workers at Stanford University first reported “picket-fence porphyrin”, having four sterically bulky groups in the structure (Figure 2)2,3. This compound stably bound O2 in absolute toluene without being oxidized to the iron(III) state. This is the first demonstration of the formation of stable O2-complex using totally synthetic compounds.
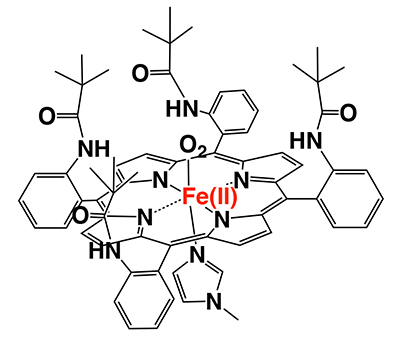
Since the report on picket-fence porphyrin, researchers have generally recognized that “to create iron-O2 complex, steric hindrance is the most important factor”. Accordingly, a lot of picket-fence porphyrin analogues have been synthesized and reported3. Indeed, these compounds form stable O2-adducts without autoxidation; however, O2-adducts could not be detected unless dissolved in absolute organic solvents.. Contamination of these compounds by only a trace amount of water was not allowed. It must be said that picket-fence porphyrin and its related compounds could not mimic the function of hemoglobin.
So, how do water molecules destabilize iron-O2 complex? Prof. Shikama at Tohoku University was concerned with this issue. He has studied the mechanism of autoxidation of hemoglobin and myoglobin (a monomer unit of hemoglobin present in muscle)1. Autoxidation of O2-heme complex in water can be accelerated by nucleophiles like water or hydroxyl anion through a mechanism of SN2-like reaction frequently occurring between organic molecules (Figure 3). Without protection from globin proteins, this reaction proceeds very rapidly. On the other hand, once globin protein covers heme in its hydrophobic heme pocket, nucleophiles hardly have any access to heme and thus autoxidation is significantly suppressed. Early synthetic porphyrins such as picket-fence porphyrins succeeded in preventing self-aggregation-promoted autoxidation, but failed in preventing autoxidation accelerated by small nucleophiles like water or hydroxyl anions. Such misdirection of the molecular design significantly delayed the development of artificially synthetic hemoglobin that can function in water as well as in living organisms.

Per-O-methylated β-cyclodextrin (2,3,6-tri-O-methyl-β-cyclodextrin, TMe-β-CD) can include tetraphenylporphyrin derivatives very specifically in water. Prof. Kano and his co-workers reported that the nano-hydrophobic environment, in which the water-soluble porphyrin can be isolated by two TMe-β-CD molecules, is very similar to that of heme in native hemoglobin.
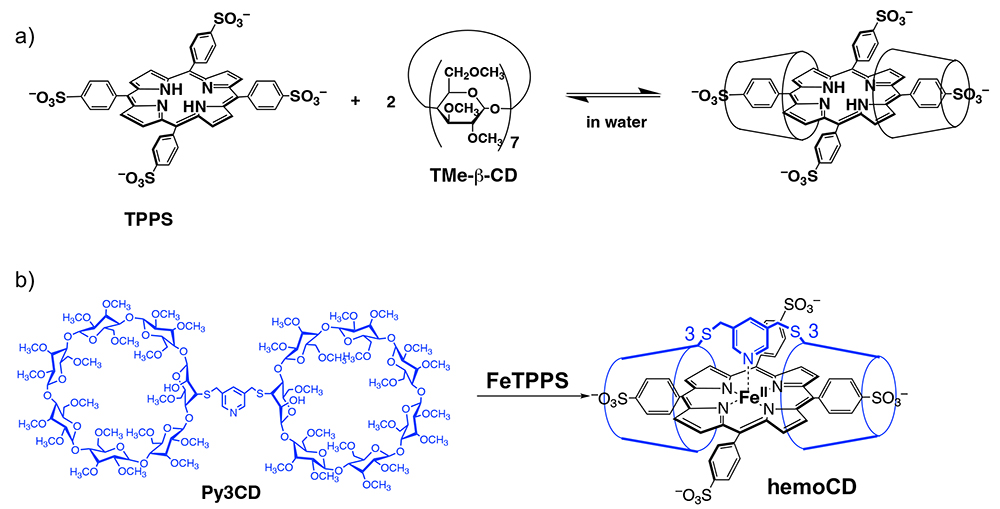
Since the discovery, Prof. Kano and I (Kitagishi) synthesized Py3CD, which is TMe-β-CD dimer linked by pyridine, and found that Py3CD formed a very stable 1:1 inclusion complex with water-soluble FeTPPS in water (Figure 4b)6,7. Furthermore, thanks to the nano-hydrophobic environment created by Py3CD, surrounding water molecules cannot approach the porphyrin core in the FeTPPS/Py3CD inclusion complex. Therefore, the inclusion complex contains a stable iron(II) complex that can bind O2 reversibly in water. This is the first and very rare example of a compound that mimics the O2-binding function of hemoglobin in water. We named this complex hemoCD and started using this complex in biological experiments.
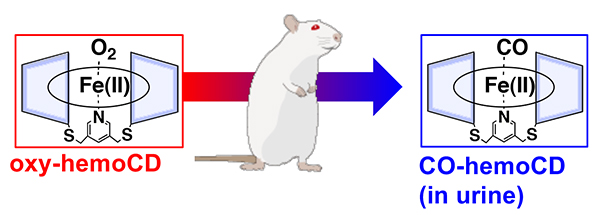
Relying on the hemoglobin-like property of hemoCD, we have tried to use hemoCD as an artificial O2 carrier in blood and thus injected rats with hemoCD. Intravenous injection of hemoCD dissolved in saline did not cause any significant damage to the rats. Notably, injected hemoCD was freely excreted in urine. Based on the color of the urine, it is not difficult to detect urinary excretion of iron(II) hemoCD without oxidation to the iron(III) state. As the red color of the urine was more vivid visually than expected for the O2-complex, we measured the spectrum of the urine. The result clearly showed that the urine contained the carbon monoxide (CO) complex of hemoCD (Figure 5)8.
The O2 binding affinity of hemoCD is comparable to that for native hemoglobin. On the other hand, the CO binding affinity of hemoCD is approximately 100-times higher than that of native Hemoglobin in its relaxed R state. The reason of such a high CO binding affinity is the hydrophobic nano-cavity provided by Py3CD, which likely traps the hydrophobic CO molecule and allows hardly any dissociation to the external water phase. Because of the extremely high CO binding affinity, hemoCD could capture endogenous CO in rats and be readily excreted through renal filtration to the urine.
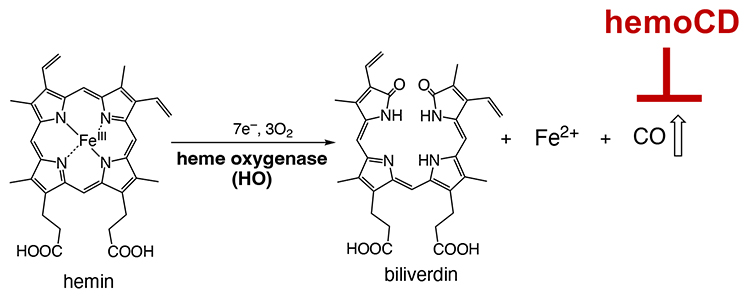
While regarded as a highly toxic gas, CO is continuously produced in the living organisms during the metabolic degradation of excess heme by heme oxygenase enzymes (Figure 6). This metabolic reaction usually occurs in living cells; CO is continuously generated in the body. Endogenously produced CO is a signal mediator that functions in vivo. However, the biological role of endogenous CO is not fully understood. We considered that we could easily prepare “CO in a pseudo knockdown state” by injecting hemoCD into animals, which could help us understand the biological roles of endogenous CO. Our study revealed that there is (1) a feedback mechanism to keep the endogenous CO level constant, which starts when the endogenous CO level is decreased by hemoCD9, (2) an increase in reactive oxygen species level upon removing intracellular endogenous CO10, and (3) a disruption of the circadian rhythm system when endogenous CO in mice is removed by hemoCD11.
Because hemoCD can remove CO from the animal body via urinary excretion, it was expected that hemoCD would act as a specific antidote to CO poisoning. To confirm this, we established a CO-poisoned mouse model with high CO-hemoglobin level and injected hemoCD into the model mice. As expected, the decrease of CO-hemoglobin level was significantly larger in the hemoCD-treated group. Furthermore, it was revealed that hemoCD decreased the level of CO in the brain, which is hard to remove by the simple O2 ventilation method13. We confirmed that hemoCD cannot penetrate blood brain barrier and thus cannot reach the brain. Therefore, hemoCD can accept CO from the brain through the gas-exchange mechanism and be totally excreted in urine.
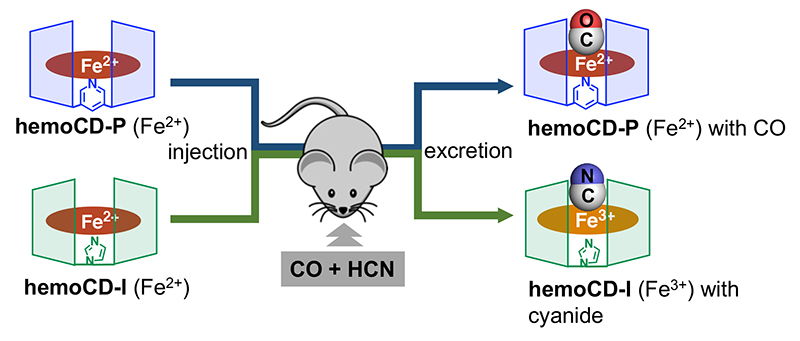
We then modeled real-fire gas poisoning. In fire accidents, not only CO but also HCN is generated as a mix of highly toxic gas components. We developed hemoCD-Twins as a joint antidote to CO and HCN. In this system, hemoCD-I, which has the CD dimer with imidazole ligand (Im3CD) to capture cyanide anion, is added to the conventional therapy with hemoCD (hemoCD-P), which has Py3CD as host molecule to capture CO (strongly). Named hemoCD-Twins (Figure 7), the mixture of hemoCD-P and hemoCD-I is injected as a single injection to remove internal CO and HCN gases. Indeed, when we injected hemoCD-Twins into the CO/NaCN dual toxicity mouse model, approximately 85% of the injected mice survived (n = 11/13) while all the uninjected mice died (n = 0/18)13. Furthermore, when real fire-gas exposure was modeled using acrylic cloth, we found that hemoCD-Twins significantly increased survival with rapid physical recovery. We believe that hemoCD-Twins will be used as a ready-to-use antidote to fire-gas poisoning.
We developed hemoCD using CDs and porphyrins as a biomimetic of hemoglobin. The artificial hemoglobin compound, hemoCD, shows reversible O2 binding in water quite like that of native hemoglobin. On the other hand, hemoCD was quite different from native hemoglobin in that it showed very high CO binding affinity and rapid urinary excretion. Our biomimetic approach yielded hemoCD with properties that were similar to but slightly different from those of hemoglobin. These properties can be used to elucidate CO biology and develop life-saving fire-gas antidotes. We intend to use our hemoCD as a ready-to-use antidote for CO and cyanide poisoning in real life scenarios.