
Mitsumasa Osada
Associate Professor, Department of Chemistry and Materials, Faculty of Textile Science and Technology, Shinshu University, Japan
He studied chemical engineering at Tohoku University, where he received his Ph.D. in 2005. He then joined the National Institute of Advanced Industrial Science and Technology (AIST), Japan as a postdoctoral fellow. He worked as an Assistant Professor at the National Institute of Technology, Ichinoseki College in 2006 and was promoted to Associate Professor in 2011. In 2007, he worked as a visiting scholar at the University of Michigan in the USA. In 2014, he moved to Shinshu University. His research interests focus on the synthesis of materials based on biomass using water. He received the Award for Encouragement of Research from the Japanese Society for Chitin and Chitosan in 2017.
Using only water as solvent for purification of chitin and preparation of chitin materials is a new green technology. We propose a new method of chitin preparation without acid/base catalysts or organic solvents that can be achieved through the regulation of water temperature and pressure.
Since the 20th century in not only the chitin industry but also the whole chemicals industry, various chemical substances have been separated, refined, and converted into materials using acid/base catalysts and organic solvents. This system can be maintained when large quantities of fossil resources are available at low cost, but in the future, the quantity of fossil resources is expected to decline and the cost of using acid/base catalysts and organic solvents is expected to rise. In addition, the dependence of the chemicals industry on fossil resources is essentially non-sustainable. Researchers and engineers living at the beginning of the 21st century have a mission to develop stable technologies that are based on those already in use. One solution to problem of technological sustainability is the use of "water" as a chemical reaction medium.
In the chitin industry, more environmentally friendly technologies for separating, purifying, and converting chitin are needed. We have been innovating technologies that utilize chitin.
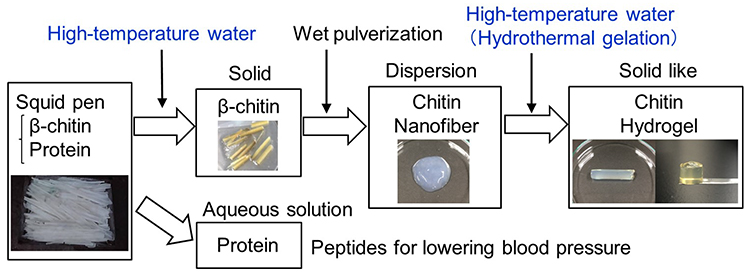
In nature, chitin is normally present combined with proteins and ash. To purify chitin, protein and ash must be removed. At present, acids and bases are used for chitin purification, but in the future, we will need chitin purification methods that require smaller amounts of acid or base, or none at all. In addition, it is important not only to use chitin but also to effectively utilize other components such as proteins and ash at the same time. Furthermore, environmentally friendly methods are required to convert purified chitin into various functional materials. Although these points are not regarded as problems at present, they will become problems. To solve these problems, we have been investigating the purification and production of chitin using only water (Figure 1)1, 2.
Water exists abundantly on the earth and is an environment-friendly substance. On the other hand, when viewed as a solvent for chemical reactions, water is not an effective reaction medium because its physical properties are limited at ambient temperature and pressure. However, by using water at high temperature and pressure (100–300ºC and 0.1–1.5 MPa) as a reaction medium, chitin can be purified and converted to materials as described later.
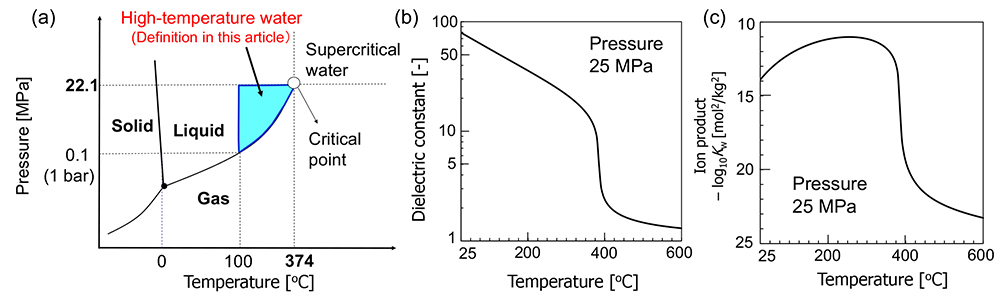
Figure 2 shows (a) the phase diagram of water, and the temperature-dependence of (b) its dielectric constant and (c) ion product.
Substances, including water, exist in three states: solid, liquid, and gas, depending on temperature and pressure. Water boils at 100ºC under 0.1 MPa (ambient pressure, 1 bar). The boiling temperature increases with increasing pressure, and the upper limit of the vaporization curve, which is the line separating the liquid state from the gas state, is the critical point. In this article, water at a temperature above 100ºC and a pressure above the saturated vapor pressure is defined as "high-temperature water".
The dielectric constant of water is large (78) at ambient temperature and pressure (25ºC and 0.1 MPa) because of the formation of hydrogen bonds between water molecules. The hydrogen bonds in the liquid phase weaken with increasing temperature and the dielectric constant also decreases. The dielectric constant of water at 250ºC is 27, which is similar to that of ethanol. Due to this change in dielectric constant, it is possible to dissolve organic compounds that do not dissolve in water at ambient temperature and pressure.
The ion product of water is Kw = [H+][OH−] = 10−14 mol2 kg−2, namely pH=7, at ambient room temperature and pressure. The ion product changes intricately with increasing temperature at 25 MPa. The dissociation of water is accelerated with increasing temperature up to around 250ºC. But water density decreases with increasing temperature above 250ºC, and the dissociation is suppressed. As a result, the level of ion product decreases above 250ºC. By changing the temperature and pressure, the concentrations of [H+] and [OH−] can be controlled, and various organic reactions proceed without acid/base catalysts.
Although energy is required to obtain a high-temperature and high-pressure reaction medium, the construction of large-scale photovoltaic power generation has progressed in recent years. This renewable energy source might enable the sustainable operation of chemical processes using high-temperature water.
Chitin has two crystalline structures, namely α-chitin and β-chitin. α-Chitin is purified from the exoskeletons of crustaceans and insects and has antiparallel molecular chains with hydrogen bonds between the chains5. On the other hand, β-chitin is purified from squid pens and tubeworms and has parallel molecular chains without significant hydrogen bonds between the intermolecular sheets. As a result, β-chitin is thermodynamically metastable compared to α-chitin6.
The squid pen contains 30 wt% β-chitin, 70 wt% protein, and less than 1 wt% ash (Figure 1). When treated with high-temperature water (150–250ºC, 30–120 min), squid pen yields β-chitin as a solid residue and protein as a water-soluble peptide. The white solid residue was confirmed to be β-chitin by X-ray diffraction and infrared spectroscopy. Using only water, the protein can be converted to a low-molecular-weight water-soluble peptide, which can be used for lowering blood pressure. High-temperature water treatment is a promising technique for the complete utilization of squid pen components, including β-chitin and protein.
The white β-chitin residue obtained above by high-temperature water treatment was disintegrated into nanofiber form (1 wt% dispersion) by wet pulverization (Figure 1). The physicochemical properties such as transmittance of the β-chitin nanofiber dispersion were almost the same as those of material produced by the traditional method using acid and base treatment7–11. The β-chitin nanofiber width observed by the use of field-emission scanning electron microscopy was also similar to the nanofiber width of material produced by the traditional method.
The chitin nanofiber dispersions easily flow when inverted and are unable to maintain their shape at low concentrations of less than 2 wt%. Self-sustaining hydrogels, which have high strength and can maintain their shape, have medical applications. In general, self-sustaining hydrogels have been prepared using additives via chemical crosslinking or immersion in alkaline solution12. However, the use of additives may impede subsequent application of the hydrogels owing to toxicity and unpredictable behavior.
On the other hand, there is a new method of preparing self-sustaining hydrogels using only water called ''hydrothermal gelation'', which is achieved by placing nanofiber dispersions under high-temperature and high-pressure conditions (Figure 1)2. The β-chitin nanofiber dispersion obtained above by wet pulverization can be converted by hydrothermal gelation (160–200ºC, 10–120 min) to a mechanically strong hydrogel that can maintain its shape. In previous studies, hydrothermal gelation of β-chitin nanofibers obtained by acid treatment13 and cellulose sulfate nanofibers14 have also been reported. The hydrothermal gelation is applicable to α-chitin2 and cellulose nanofibers obtained by wet pulverization15, and TEMPO-oxidized cellulose nanofibers16.
In addition to hydrogel, a sponge material (aerogel) can also be obtained by freeze-drying a chitin nanofiber dispersion17.
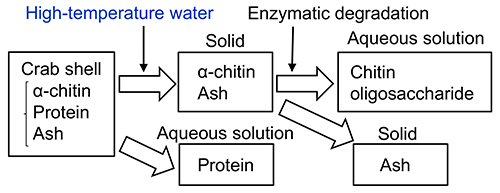
The β-chitin purification from squid pen was mentioned above. For crab shells, proteins are also hydrolyzed after high-temperature water treatment to low-molecular-weight water-soluble peptides, but a mixture of α-chitin and ash remains as a solid residue. When this mixture of α-chitin and ash is used as a substrate for enzymatic degradation, oligosaccharides of chitin and N-acetyl glucosamine can be obtained. The enzymatic degradation does not proceed without high-temperature water pretreatment. During high-temperature water treatments, the mean molecular weight of α-chitin decreased and the distance between chitin chains increased due to the weakening of hydrogen bonds19–24.
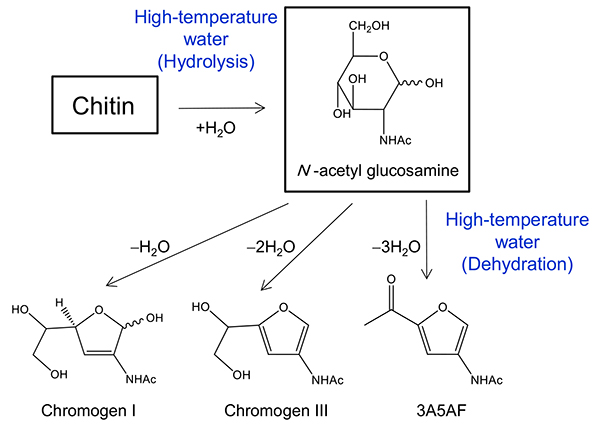
As shown in Figure 4, the conversion of chitin and N-acetyl glucosamine into useful nitrogen-containing compounds has attracted attention29–31. In previous studies, it has been reported that N-acetyl glucosamine and chitin oligosaccharides can be treated in a borate solution at 100ºC for 2 hours to synthesize compounds with acetamide groups, which are useful as pharmaceutical raw materials31. However, the separation of the boric acid catalyst is a problem when using the products as pharmaceutical raw materials.
We investigated the conversion of chitin and N-acetyl glucosamine to nitrogen-containing compounds in high-temperature water without acid catalyst. As a result, we showed that the hydrolysis of chitin and dehydration of N-acetyl glucosamine proceeded in high-temperature water without any catalyst, and nitrogen-containing compounds could be obtained. The important feature of the process in high-temperature water is that it can be switched from "hydrolysis" to "dehydration" by changing the reaction conditions of water.
The method using only water described in this article does not necessarily have higher product purity or yield than methods currently in practical use. On the other hand, since the 20th century, the quality of not only chitin products but also chemicals as a whole has been excessive because of low-cost raw materials and energy derived from inexpensive fossil resources. Until now, the main focus has been on the production of new materials and products of the best possible quality. We believe that the chemicals industry needs to rethink its product quality requirements to meet the future challenge posed by lack of raw materials and energy from fossil resources. The chemicals industry might also want to consider whether high-quality traditional raw materials are really needed to achieve the functionality and properties of the current final products. We believe that the technology using only water introduced here will play an important role in the production of chitin-derived products that meet amended quality requirements.