Tomohiko Ishii
Professor at the Faculty of Engineering and Design, Kagawa University, and International Institute of Rare Sugar Research and Education, Kagawa University
Career: Tomohiko Ishii received his Doctor of Science degree from the Department of Chemistry, Graduate School of Science and Engineering, Tokyo Institute of Technology in 1996. He became an assistant professor at the Faculty of Science and Engineering, Iwaki Meisei University in the same year, and then an assistant professor at the Graduate School of Science, Tokyo Metropolitan University in 1997. In 2002, he was appointed an assistant professor at the Faculty of Engineering, Kagawa University, later an associate professor and a professor there, and in 2018, a professor at the Faculty of Engineering and Design, Kagawa University.
He specializes in solid state physics and quantum materials science. He is fascinated by the depth of the subject of rare sugars and is struggling daily to investigate the detailed crystal structure of rare sugars to know their electronic structure.
This article focuses on the crystal structures of monosaccharides and rare sugars and explores how the molecules interact with each other (i.e., intermolecular interaction) and how this interaction affects the morphology and properties of the crystals. Examining these points reveals the characteristics of a substance. There are molecules that have very similar molecular structures but very different crystal structures; for example, D-glucose and D-allose, or D-fructose and D-allulose. I am attempting to clarify these structural differences and to elucidate the underlying mechanisms of hydrogen bond formation and intermolecular interactions. I am also investigating the potential for novel applications of rare sugar crystals, and here I present an approach on how to control the crystal structure to obtain a specific physical property. In addition, this article provides a new perspective on the longstanding mystery of whether the crystal structure of monosaccharides can be controlled. I hope that through this article, readers will gain a concrete understanding of research progress in the application of rare sugar crystals and will be inspired to learn how the knowledge of crystal structures can contribute to materials science, medicine, agriculture, and other fields.
An organic crystal is a solid-state substance formed by molecules arranged in a regular pattern (Fig. 1). Crystal formation of organic molecules is greatly influenced by molecular interactions, such as hydrogen bond, van der Waals, and hydrophobic interactions. These interactions are responsible for a crystal's unique morphology and physical properties (e.g., giant optical rotation, spontaneous polarization, magnetic behavior, and difference in melting points). Monosaccharides, such as D-glucose, have many hydroxy groups (-OH) and are particularly affected by intermolecular interactions through hydrogen bonds. Hydrogen bonds are weak intermolecular attractions, but they serve to hold the monosaccharide molecules firmly together and maintain the crystal stability. Monosaccharides are generally highly soluble in water, and their crystals can be readily precipitated from their aqueous solutions. The resulting crystals are mostly transparent and colorless, and their shape is needle- or plate-like. The crystals are soft and easily absorb water; in particular, monosaccharides such as glucose, fructose, and allose may form crystals in which water molecules are incorporated as crystal solvents. Another characteristic of monosaccharide crystals is that most of them are not polymorphic. Although there are a few exceptions such as allose, most monosaccharides have only one type of crystal structure.
Within the field of monosaccharides, the study of rare sugars has progressed tremendously since the publication of the mass synthesis strategy “Izumoring” by Ken Izumori (see an introductory video of Izumoring at the following
URL: https://www.eng.kagawa-u.ac.jp/~tishii/pov/Images/Izumoring.mp4). For example, in the fields of medicine, pharmacy, and agriculture, rare sugars have been reported to: inhibit blood sugar level elevation, promote fat burning, inhibit cancer cell growth, behave as edible pesticides and low-calorie sweeteners, improve the intestinal environment, act as antioxidant and anti-inflammatory agents, and promote calcium absorption.
For further progress of rare sugar research and the development of new applications, it is necessary to focus on the areas of study where little research has been done so far; for example, the possibility of application as industrial materials should be considered. To use rare sugar crystals as novel materials with new functions, the crystals need to have targeted physical properties (e.g., electrical conductivity, magnetic behavior, photoresponsiveness), and to achieve this, the molecules in the crystal must be properly oriented. To be more specific, sugar molecules have charge bias (this is called dipole moment). This is a state in which there are electrically positive and negative poles, like the N and S poles of a magnet. If this electrical orientation can be fully aligned, a giant circularly polarized light-emitting device can be constructed. Besides, if the spin moments of sugar molecules can be aligned in the same direction, creating ferromagnetic magnets will be possible.
A sugar molecule originally has many asymmetric carbons, and the molecule as a whole has a unique optical rotation. In aqueous solution, the orientation of the molecules changes randomly, but even in such state, they exhibit optical rotation; this property is used to measure sake meter value (an indicator for the taste of Japanese sake) and sugar content. If the optical rotation of sugar molecules in a crystal could be aligned in one direction, the crystal might possibly be used as an optical filter with the property of giant optical rotation.
In reality, however, it is unfortunate that orienting molecules in organic crystals as intended is extremely difficult to do. Not only for monosaccharides, but also even for general organic molecules and inorganic compounds, a method for designedly aligning molecules in single crystals has yet to be established. The purpose of this article is to introduce the crystal structures of various types of natural monosaccharides, rare sugars, and their derivatives that have been studied in our group, and to present the fact that the crystal structures of sugars can be quite different even among those with very similar molecular structures. Furthermore, this article describes how we are still working to find a way to orient sugar molecules as desired. Chapter 2 presents the differences in crystal structure among hexoses. Chapter 3 describes crystal structures of hydrated compounds, and Chapter 4 introduces racemic crystals. Chapter 5 presents alkyl sorboside crystals that have antimicrobial properties, and in Chapter 6, I describe future approaches for predicting and controlling crystal structures.
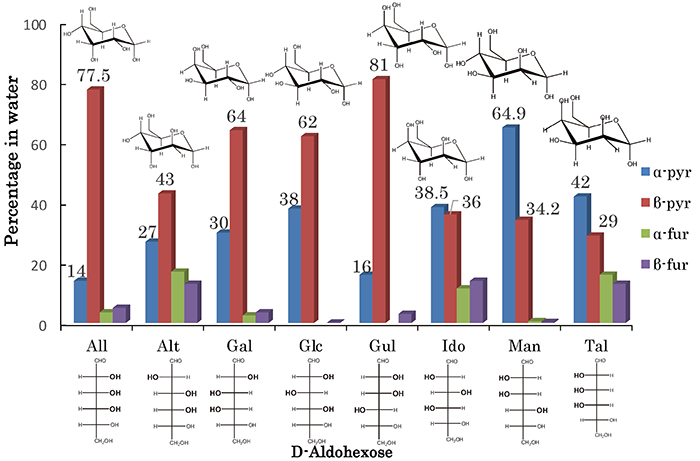
This chapter introduces the difference in crystal structure among hexoses, and how the differences affect their physical properties. Hexoses are important molecules involved in energy metabolism. Among monosaccharides, hexoses having the general chemical formula C6H12O6 are widely found in nature. Hexoses such as glucose are central molecules in energy metabolism and are involved in the glycolysis and citric acid cycles. In addition, as components of polysaccharides, hexoses are involved in the synthesis of cell walls and energy storage materials as well as in intercellular recognition and signal transduction. Because of their diversity and biological importance, hexoses have been the subject of very active research and have been applied in a variety of fields, including medicine, biotechnology, and food science. In particular, D-aldose, which has an aldehyde group (-CHO), has eight stereoisomers (epimers) as shown in Fig. 2. These eight stereoisomers exist in aqueous solution as mixtures of four cyclic tautomers (anomers) (α-pyranose, β-pyranose, α-furanose, and β-furanose) and rarely in chain form (<1%).
This article mainly focuses on the difference in the structures of hexose crystals, but in order to help many readers understand the crystal structures of sugars, I first introduce the basic concepts of crystallography in the following two paragraphs. Readers who already have a good knowledge of crystal structures can skip these paragraphs.
First of all, a crystal is a solid in which molecules and atoms are arranged according to a certain rule. Many sugars tend to be in a crystalline state, just like salt and ice, and form visible crystals. There are various types of arrangements of molecules or atoms that make up crystals, and are referred to as regularities within the crystal system. Examples of crystal systems include the orthorhombic and triclinic systems; the orthorhombic system has cuboid-shape, and the triclinic system has parallelepiped-shape unit cells (the smallest unit of a crystal having a repeating periodic structure in which molecules or atoms are regularly arranged). Furthermore, the concept of a space group is important to understand crystal symmetry. Crystals often have point, plane, or rotational symmetry due to the regular arrangement of atoms. The space group is a mathematical description of the symmetry of the molecules or atoms in a crystal.
For example, the space group P212121 appears later in this article; the meaning of this notation is that this crystal belongs to the orthorhombic crystal system and has parts where the molecules form a structure like a helix in three-dimensional space (which is called the “21 axis”). This helix-like arrangement is made by repeating the combination of moving the molecules slightly and then rotating them 180 degrees. Since it is difficult to explain this in words only, I prepared an illustrative video. I hope the readers will visit the website at the following URL, which introduces the structure of the space group P212121, in particular the symmetry operation of the 21 screw axis, using D-allose as an example (https://www.eng.kagawa-u.ac.jp/~tishii/pov/Images/P212121All.mp4). There are also other types of symmetry operations that result in a very regular arrangement of molecules, thus preserving the overall stability of the crystal.
Differences in crystal structure have a significant impact on the physical and chemical properties of substances. For example, even substances with the same chemical formula may have different solubility, melting point, optical properties, and other characteristics if they have different crystal structures. Therefore, accurate understanding and control of crystal structures is very important in the fields of materials, pharmaceutical, and agricultural sciences.
This article focuses on the crystal structures of monosaccharides, particularly the diversity in the crystal structure of hexoses. Hexoses have many structural isomers. Here I will show how these isomers differ greatly in their crystal structures, even when there are only minor differences in their molecular structures.
The example here is two aldohexoses, D-glucose and D-allose, which have very similar molecular structures. Fig. 3 shows the typical molecular structure of D-glucose (on the left) and that of D-allose (on the right) as β-D-pyranose forms; the abundances in aqueous solution are 62% and 77.5%, respectively. These two molecules differ only in the orientation of the OH group at the C-3 position, and the rest and most of the molecular structure is identical. However, the crystal structures of these two sugar molecules differ greatly. Fig. 4 shows the crystal structures (molecular packing diagrams); D-glucose is on the left and D-allose is on the right. Both are a-axis views. The six-membered rings of D-allose are in orderly alignment, whereas those of D-glucose are tilted.
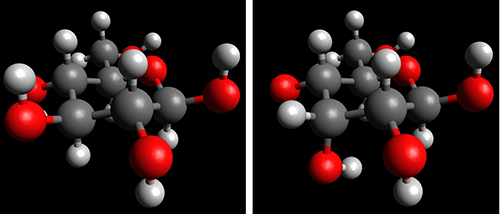
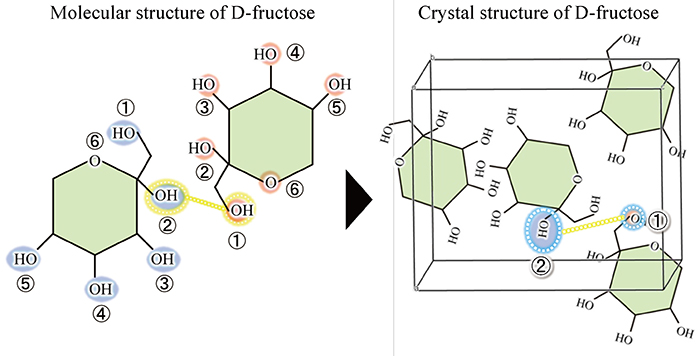
In addition to D-aldose, other sugars have extremely similar molecular structures but very different crystal structures. The next example is D-fructose and D-allulose, which are typical D-ketoses with a ketone group (-C=O). As shown in Fig. 5, the only difference between the molecular structures of D-fructose and D-allulose is the orientation of the OH group at the C-3 position. Nevertheless, the crystal structures of these two sugars are very different. For example, in D-fructose, the hydrogen bond is formed between the OH group at the C-2 position (hereafter referred to as O2) and O1, whereas in D-allulose, the hydrogen bond is formed between O2 and O4. Of note, the difference in the molecular structures between D-fructose and D-allulose is the orientation of the OH group at the C-3 position, but the difference in the hydrogen bond in the crystals is seen at other OH groups (O2, O1, or O4), not at O3.
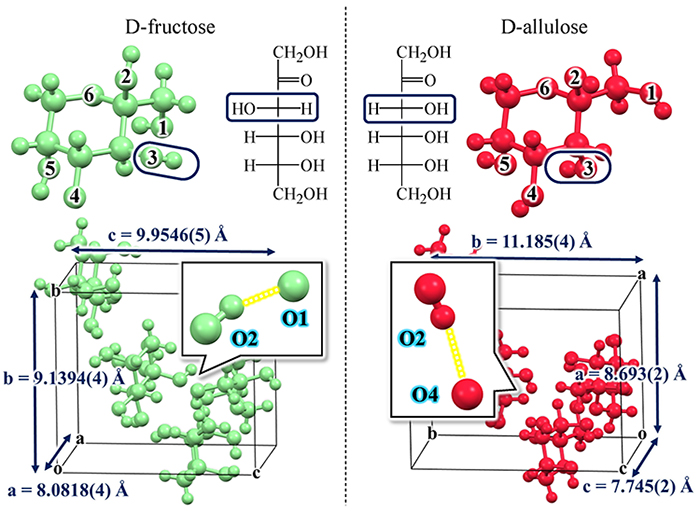
Another notable point is that the positions at which hydrogen bonds occur are not determined randomly. Specifically, as shown in Fig. 6, D-fructose has five hydrogen-donor OH groups (light blue) and six hydrogen-acceptor oxygen atoms (red). In other words, there are 5 × 6 = 30 possible hydrogen bond pairs. However, the main hydrogen bond is not randomly selected from among these 30 possible combinations; in fact, only O2 → O1 is selected for D-fructose and only O2 → O4 for D-allulose. In the case of D-fructose, this main O2 → O1 hydrogen bond is repeated among the molecules to form a larger crystal.
Incidentally, organic compounds with chiral structures, such as D-hexose, often have a crystal form with symmetry of the space group P212121 seen in the orthorhombic system (Fig. 7). This structure has a cuboid unit lattice and 2-fold screw axes (21 axes) for each of the x, y, and z axes. The screw axis implies a simple rotational operation; for example, this operation turns the D-form over and it remains the D-form (imagine a right hand turning over). The relative coordinates (x, y, z) of the independent atoms in the unit lattice are duplicated by the P212121 symmetry operations into (1/2 + x, 1/2 – y, –z), (–x, 1/2 + y, 1/2 – z), and (1/2 – x, –y, 1/2 + z), resulting in a state with totally four atoms in a unit lattice. Note that in the field of crystallography, the number of molecules in a unit lattice is indicated by the parameter Z (Z = 4).
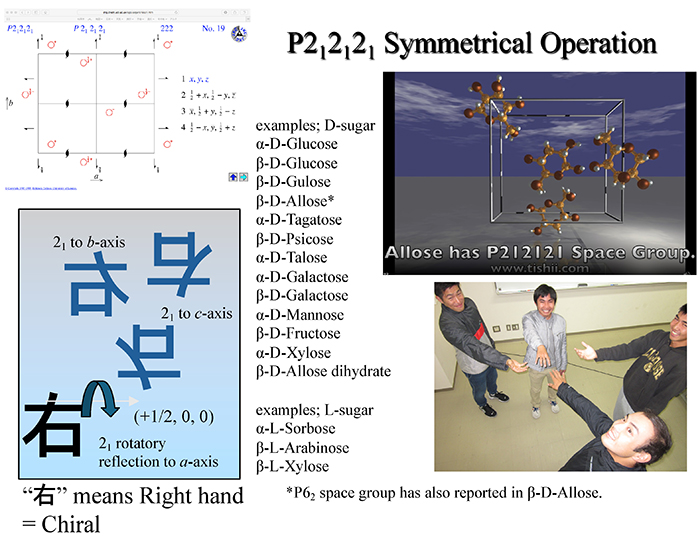
As shown in Fig. 7, many monosaccharides and their derivatives have been reported to possess this P212121 space group, regardless of whether they are D- or L-forms, aldose or ketose, hexose or pentose, and anhydrous or hydrous. Table 1 is the summary, although only for hexoses, of the results reported so far on the characteristics of the crystal structures. Here, CSD ID is the identification code in the Cambridge Structural Database (CSD). CSD is a database of crystal structures of organic and organometallic molecules and has been accumulating data since 1965; the Cambridge Crystallographic Data Centre (CCDC) in Cambridge, UK, is solely responsible for data registration and processing.
As can be seen in Table 1, many monosaccharides have the P212121 space group. The exception is D-altrose. The D-altrose has the trigonal P32 space group instead of the orthorhombic one. Despite the follow-up experiments conducted by research groups around the world, including ours, no orthorhombic single crystals of altrose have been obtained to date. Next, D-mannose also has the unique property of being difficult to form single crystals. The D-mannose, which is abundant in nature and is found in konjac, corn, aloe, and seaweed is not a rare sugar and is inexpensive and readily available as a reagent. Therefore, structural organic chemists around the world have tried to analyze the single crystal structure of D-mannose. However, high-quality single crystals of D-mannose have not been obtained so far. The reasons for the existence of these two distinctive crystal structures, D-altrose and D-mannose, are still unclear; this is an issue to be addressed in the future study. No one in the world, including our group, has yet succeeded in single crystal structure analysis of D-idose. We hope to succeed in measuring it as soon as possible for the sake of systematic discussion of the crystal structure of hexoses.
| Sugar | Space Group | Cell Parameters (Å,º) |
Cell Volume (Å3) |
Z | CSD ID | Ref. |
| β-D-Allose | P212121 | a 4.918(1) b 11.925(2) c 12.805(2) | 750.977 | 4 | COKBIN | [1] |
| β-D-Allulose (Psicose) |
P212121 | a 8.693(2) b 11.185(4) c 7.745(2) | 753.056 | 4 | OGETAW01 | [2] |
| β-D-Altrose | P32 | a 7.1749(13) b 7.1749(13) c 12.7415(15) γ 120 |
568.045 | 3 | EFUWEI | [3] |
| β-D-Fructose | P212121 | a 8.088(2) b 9.204(4) c 10.034(15) | 746.951 | 4 | FRUCTO11 | [4] |
| α-D-Galactose | P212121 | a 5.8999(8) b 7.8433(12) c 15.685(4) | 725.818 | 4 | ADGALA03 | [5] |
| β-D-Galactose | P212121 | a 12.655(2) b 7.771(1) c 7.701(1) | 757.332 | 4 | BDGLOS01 | [6] |
| α-D-Glucose | P212121 | a 10.360 b 14.840 c 4.970 | 764.100 | 4 | GLUCSA | [7] |
| β-D-Glucose | P212121 | a 9.205(4) b 12.640(5) c 6.654(3) | 774.201 | 4 | GLUCSE01 | [8] |
| β-D-Gulose | P212121 | a 7.0800(3) b 9.8644(3) c 10.6156(4) | 741.393 | 4 | CIVVAG | [9] |
| D-Idose | N.D. | N.D. | N.D. | |||
| α-D-Mannose (*) | P212121 | a 23.447(8) b 9.460(2) c 6.891(1) | 1528.480 | 8 | ADMANN | [10] |
| β-D-Mannose (*) | P212121 | a 5.62999(16) b 7.5401(2) c 18.0919(5) | 768.014 | 4 | OKEZAH10 | [11] |
| α-L-Sorbose | P212121 | a 6.535(4) b 18.069(7) c 6.305(4) | 744.500 | 4 | SORBOL | [12] |
| α-D-Tagatose | P212121 | a 6.237 b 6.529 c 17.757 | 723.089 | 4 | TAGTOS | [13] |
| α-D-Talose | P212121 | a 8.101(2) b 12.126(2) c 7.655(1) | 751.972 | 4 | ADTALO01 | [14] |
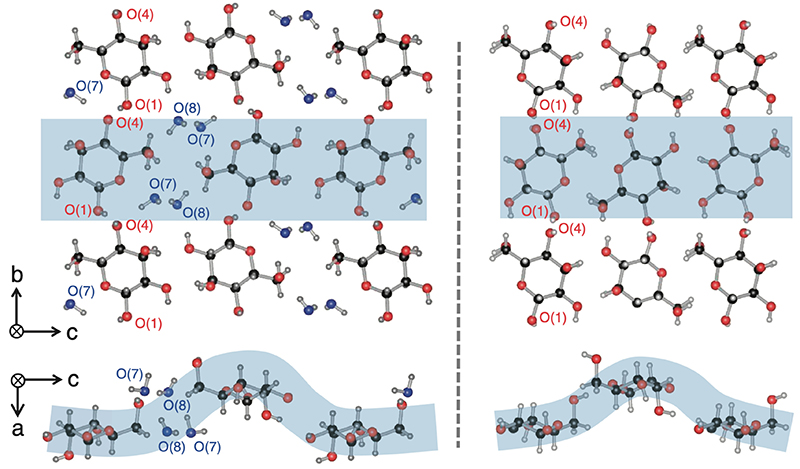
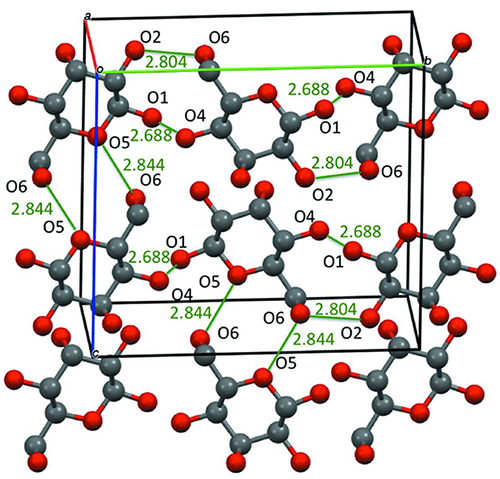
Monosaccharide crystals are soft and easily absorb water. In particular, monosaccharides such as glucose, fructose, and allose may form crystals in which water molecules are incorporated as crystal solvents. Table 2 shows two examples of such crystals. D-glucose monohydrate crystals incorporate one water molecule per D-glucose molecule. As a result, the symmetry decreases from the highly symmetric P212121 space group to the monoclinic P21. On the other hand, D-allose dihydrate incorporates two water molecules per D-allose molecule. Nevertheless, the space group of the crystal remains highly symmetric P212121. The cell parameters for anhydrous and hydrous D-allose are as follows: a = 4.918(1) Å, b = 11.925(2) Å, and c = 12.805(2) Å for anhydrous; a = 4.81148(10) Å, b = 12.6487(3) Å, and c = 15.6012(4) Å for hydrous. Comparing these two forms, only the width in the c-axis direction was larger by about 3 Å in the hydrous form. Fig. 8 shows the crystal structure of D-allose dihydrate. The left side is the crystal structure of the hydride, and the right side is that of the anhydride. As can be seen by comparing these two structures, two water molecules (O7 and O8) are inserted in the c-axis direction while the six-membered rings of D-allose remain aligned in an orderly manner; as a result, the crystal structure keeps its high symmetry and is extended only in the c-axis direction. This indicates that D-allose has the potential to readily adsorb and desorb water and could be used in applications as a humectant.
| Sugar | Space Group | Cell Parameters (Å,º) |
Cell Volume (Å3) |
Z | CSD ID | Ref. |
| α-D-Glucose monohydrate | P21 | a 8.840 b 5.100 c 9.690 β 98.25 | 432.343 | 2 | GLUCMH | [15] |
| β-D-Allose dihydrate | P212121 | a 4.81148(10) b 12.6487(3) c 15.6012(4) | 949.473 | 4 | IHOTUW | [16] |
Monosaccharides generally have two enantiomers, D- and L-forms. The following explanation assumes that the D-form is a baseball glove for the right hand, and the L-form is that for the left hand (Fig. 9). If only the gloves for the left hand were abundant, the way to arrange them to fill the space as densely as possible would be to align them while rotating them by 90°, as in the P212121 space group. Crystals formed in this way are called chiral crystals. In contrast, if a large and equal number of right- and left-hand gloves were present, the space would be filled more densely, because the unfilled space that exists in the chiral crystal is completely eliminated by the right- and left-hand gloves complementing each other. Crystals formed in this way are called racemic crystals. In organic compounds, racemic crystals generally fill the space more densely than chiral crystals, and therefore, racemic crystals tend to have a smaller unit volume and larger density. This tendency is called Wallach's rule17.
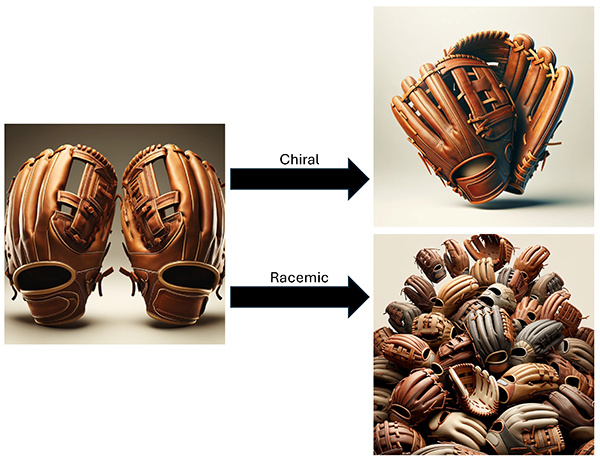
Fig. 10 shows the structure of the D,L-allose crystal formed with a D-allose to L-allose ratio of 1:1. This is a racemic crystal; however, the term racemic crystal may have a broad meaning, and for example, it may refer to a mere mixture of the D- and L-forms. Here, this article does not consider mere mixtures or partial alternating multilayers of D- and L-forms, and in this article, supramolecular crystals with a pure superlattice structure are referred to as supramolecular rare sugars, which is the term coined by Ken Izumori. At the Rare Sugar Congress 2011, the International Society of Rare Sugars (ISRS) deemed supramolecular rare sugars to be one of the official classifications of rare sugars, leading to a revision of the previous definition of rare sugars (the websites at the URLs below provide videos to introduce the supramolecular rare sugar structure of D,L-allose).
https://www.eng.kagawa-u.ac.jp/~tishii/pov/Images/DLAllose.mp4
https://www.eng.kagawa-u.ac.jp/~tishii/pov/Images/DLAllose2.mp4
On a different note, supramolecular rare sugars that do not satisfy Wallach’s rule were obtained. Fig. 11 summarizes the crystal structures of the D-chiral and D,L-racemic supramolecular forms of allulose, allose, and fructose. Allose and fructose follow Wallach’s rule; that is, the D,L-form has smaller volume (= higher density) than the D-form (as fructose has a Z value of 2 for the D,L-form, the volume must be doubled for comparison). In contrast, allulose was found not to follow Wallach’s rule; the D,L-form of allulose has larger volume (= lower density) than the D-form. The reasons for this are as follows. In an allulose molecule, the OH groups at the C-3 and C-5 positions are both axial, and the intramolecular hydrogen bond between them generates a dipole moment. As shown in Fig. 12, this dipole moment is cancelled by the 2-fold screw axis of P212121 in the D-chiral form. On the other hand, in the D,L-racemic supramolecule form, the crystal system remains orthorhombic, but the space group becomes Pna21, which results in all dipole moments being aligned in one direction. This finding implies that the interaction between the dipole moments expands the volume of the unit lattice in the D,L-racemic supramolecule.
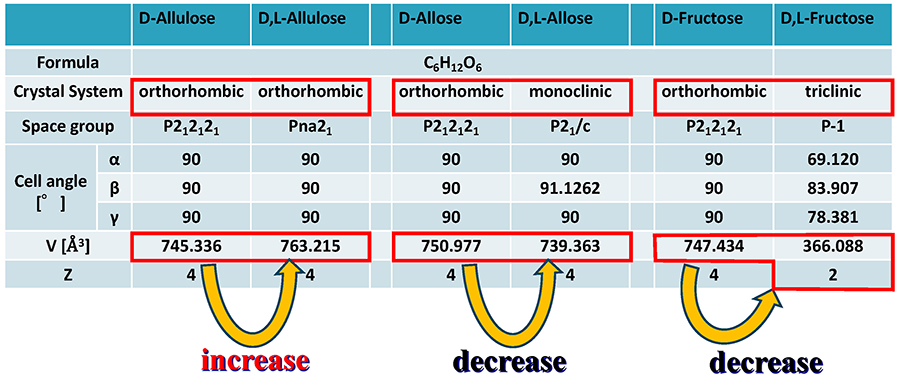
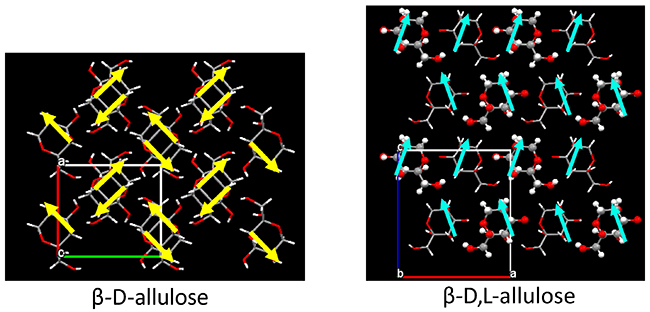
Note that this result can be considered an example of achieving our goal of aligning rare sugar molecules in one direction. As mentioned above, if the dipole or spin moments can be aligned in the same direction, it will be possible to create giant circularly polarized light-emitting devices or ferromagnetic magnets. Therefore, by constructing supramolecular rare sugar crystals, we succeeded in finding the possibility of controlling molecular orientation, which could not be achieved by monosaccharide chiral crystals alone. Other crystal structures of supramolecular rare sugars reported so far are summarized in Table 3.
| Sugar | Space Group | Cell Parameters (Å,º) |
Cell Volume (Å3) |
Z | CSD ID | Ref. |
| β-D,L-Allose | P21/c | a 4.98211(10) b 12.5624(3) c 11.8156(3) β 91.1262(14) |
739.363 | 4 | QORHUC | [18] |
| β-D,L-Allulose | Pna21 | a 11.2629(5) b 5.3552(3) c 12.6538(6) | 763.215 | 4 | XULKIA | [19] |
| α-D,L-Sorbose | P21/a | a 12.8152(3) b 6.29489(13) c 18.9482(4) β 108.3472(12) |
1450.85 | 8 | HELYOP | [20] |
| β-D,L-Fructose | P-1 | a 5.43124(19) b 7.2727(3) c 10.1342(4) α 69.120(2) β 83.907(2) γ 78.381(2) |
366.09 | 2 | NUPSUO | [21] |
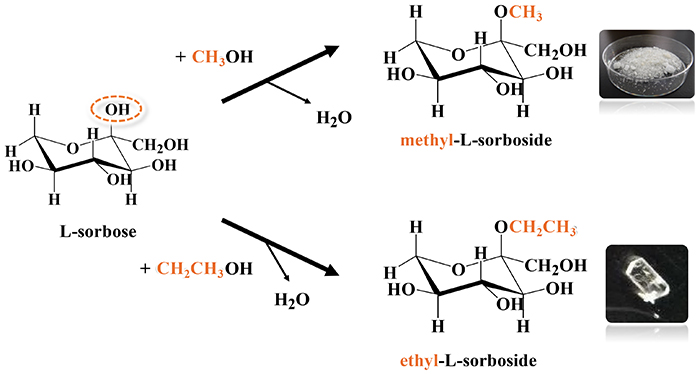
In collaboration with FUSHIMI Pharmaceutical Co., Ltd., we analyzed the structure of single crystals of sorboside compounds, which are derivatives of sorbose. Fig. 13 shows the synthetic scheme. By reacting sorbose with methanol or ethanol, the OH group at the C-2 position can be replaced with a methoxy or ethoxy group. Among the compounds thus obtained, especially an L-form alkyl-L-sorboside has selective antimicrobial activity. Selective antimicrobial activity is the action of inhibiting the growth of only certain harmful microorganisms while protecting beneficial ones and is not the action of indiscriminately killing all microorganisms. This concept is particularly important for maintaining the flora of the human body (protecting the microflora, the intestinal flora, etc.) and the microbial environment in soil and water. Table 4 summarizes the crystal structures of the methyl and ethyl forms of alkyl-L-sorboside. (The website at the following URL provides a video to introduce the structure of ethyl-L-sorboside:
https://www.eng.kagawa-u.ac.jp/~tishii/pov/Images/ELS.mp4.)
| Sugar | Space Group | Cell Parameters (Å,º) |
Cell Volume (Å3) |
Z | CSD ID | Ref. |
| Methyl-α-L-sorboside | P1 | a 6.7320(5) b 7.7574(5) c 10.6128(8), α 82.458(6) β 72.596(5) γ 65.476(5) |
481.13 | 2 | LAMPAU | [22] |
| Ethyl-α-L-sorboside | P212121 | a 6.8203(8) b 8.6934(10) c 15.865(2) | 940.63 | 4 | EJAKAE | [23] |
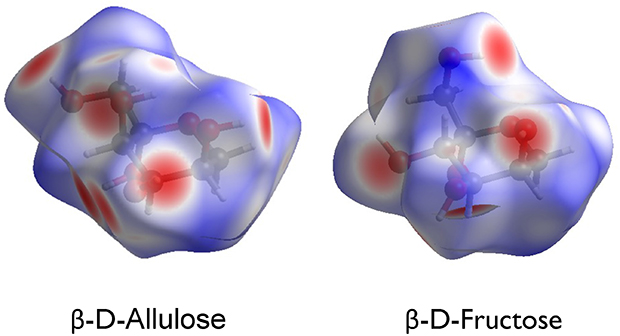
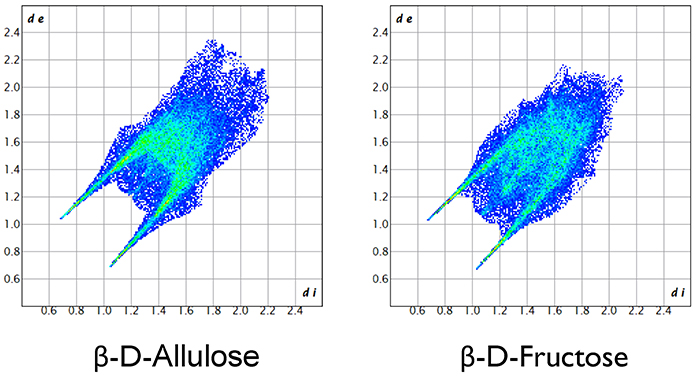
This article introduced crystal structures of monosaccharides, especially hexoses. Hexoses with the chemical formula C6H12O6, whether aldose or ketose, are merely structural isomers, and their molecular structures are very similar to each other. Nevertheless, the crystal structures differ significantly. At present, it is difficult to control the crystal structure as intended, not only in monosaccharides, but in all organic and inorganic compounds. However, we are still exploring by various means a way to manipulate crystal structures of monosaccharides. Specifically, our methods include Hirshfeld surface analysis shown in Fig. 14 and the 2D fingerprint plot analysis shown in Fig. 15. I will not explain the details of each analysis in this article because expertise in crystallography is required for a full understanding, but by using these methods, intermolecular interactions between two sugar molecules can be discussed qualitatively and quantitatively. Furthermore, as a unique effort, we are performing DV-Xα molecular orbital calculations to quantitatively investigate hydrogen bonds between sugar molecules. In the future, we would like to further elucidate long-standing mysteries in the crystal structure of monosaccharides, making full use of not only electronic structure calculations but also material informatics methods, including machine learning and AI calculations.