
Koichiro Hayashi
Associate Professor, Faculty of Dental Science, Kyushu University
Koichiro Hayashi obtained his Ph.D. in Engineering from Nagoya University in 2010. He served as an Assistant Professor at Tokushima University and later at Nagoya University. Since 2017, he has been affiliated with Kyushu University’s Faculty of Dental Science. His research specializes in biomaterials, hard and soft tissue regeneration, and nanomedicine.
Cartilage is a tissue that does not regenerate itself naturally, and the number of patients with osteoarthritis continues to increase. Current treatment methods have limitations, making the development of new regenerative technologies an urgent task. The author has developed a wood-derived sponge hydrogel that retains the skeletal structure of wood while controlling the amount of hydrogen bonding between cellulose molecules, resulting in stiffness and water content equivalent to that of articular cartilage. Furthermore, this sponge hydrogel exhibits higher compressive strength and better shape recovery (cushioning effect) than cartilage. In vitro tests showed that the hydrogel was non-toxic and promoted the chondrogenic differentiation of human mesenchymal stem cells. In vivo studies demonstrated that after transplantation into a rabbit femoral cartilage defect, cartilage extracellular matrix formed within four weeks, and the defect was completely repaired with hyaline cartilage by week 12. This sponge hydrogel has the potential to overcome the challenges of current treatments and serve as a sustainable cartilage regeneration material derived from forest resources.
Cartilage does not regenerate naturally, and the number of patients with cartilage damage is extremely high. In particular, osteoarthritis (OA), which results from the gradual wear of knee cartilage with aging, affects 25.3 million people in Japan and 528 million people worldwide. According to domestic statistics, less than 30% of patients with symptomatic arthritis receive medical treatment, while over 35% endure pain without treatment, suggesting that the potential number of patients exceeds 30 million. With the aging population increasing globally, more than 20% of the population in major countries, including the US and China, will be elderly by 2035, further increasing the number of patients. Moreover, osteoarthritis is recognized as a specific disease that qualifies for long-term care insurance, making joint disorders the leading cause of illness requiring assistance. Therefore, developing materials and technologies to regenerate and protect cartilage is crucial for improving patients' quality of life (QOL) and reducing medical and nursing care costs.
Current treatments for osteoarthritis include total knee arthroplasty, high tibial osteotomy, bone marrow stimulation techniques, and osteochondral graft transplantation. However, each of these has limitations. Total knee arthroplasty restricts mobility and affects daily life. High tibial osteotomy is ineffective in severe cases and does not achieve true cartilage regeneration. Bone marrow stimulation techniques result in fibrocartilage, which is functionally inferior to hyaline cartilage. Osteochondral graft transplantation sacrifices the patient’s healthy cartilage. Autologous chondrocyte implantation is applicable to correct traumatic cartilage defects and osteochondritis dissecans but not osteoarthritis. Moreover, cell-based therapies are costly and cannot be used in load-bearing areas, necessitating a supportive scaffold material.
Hydrogels are widely studied as biomaterials for cartilage tissue engineering because of their high water content (60–85%), similar to that of cartilage. Previous studies suggested that adjusting the stiffness of hydrogels to 80 kPa could induce chondrogenic differentiation of mesenchymal stem cells. However, this approach failed to regenerate uniform cartilage tissue and lacked clinical efficacy. While combining hydrogels with cells has been explored, cartilage regeneration using hydrogels alone is preferred due to challenges in cell sourcing, functionality, high culture costs, contamination risks, spontaneous mutations, malignancies, and immune rejection.
A biomimetic approach, synthesizing hydrogels with mechanical properties similar to those of cartilage, may achieve cartilage regeneration without cells. Since articular cartilage has a stiffness of 200–900 kPa, such hydrogels must be 2.5–11 times stiffer than conventional 80 kPa hydrogels. Additionally, they should resemble natural cartilage in chemical composition and water content and possess sufficient mechanical strength to withstand loading.
Wood has a high load-bearing capacity and a porous structure that could facilitate cell infiltration. However, unmodified wood is unsuitable as a cartilage regeneration material because of its much greater stiffness and different chemical composition. By removing components from cellulose and replacing them with cartilage-like elements, a wood-derived hydrogel mimicking cartilage could be synthesized. The rigidity of cellulose results from strong hydrogen bonds, which can be weakened by introducing molecules that modify its interactions, achieving a flexible structure comparable to that of cartilage.
Forest resources play a crucial role in environmental sustainability, and the recycling of natural materials is a global priority. Utilizing thinning wood and waste wood to create cartilage-regenerative hydrogels addresses both health and environmental issues. However, research on using wood for tissue engineering is currently non-existent.
The author has developed a method for synthesizing a hydrogel product that retains the wood's skeletal structure, removes lignin, inserts molecules to control hydrogen bonding between cellulose microfibrils, and has the same stiffness, strength, and water content as cartilage. The cartilage regenerative function of this hydrogel has been demonstrated1.
The wood-derived sponge hydrogel is synthesized as follows (Figure 1): 1) removal of lignin from wood; 2) insertion of citric acid (CA) between cellulose microfibrils (CA has multiple hydrogen bonding sites and is essential for energy production); and 3) insertion of N-acetylglucosamine (NAG) between cellulose microfibrils (NAG is a precursor of glycosaminoglycans, a promotor of their synthesis, and inhibitor of their degradation).
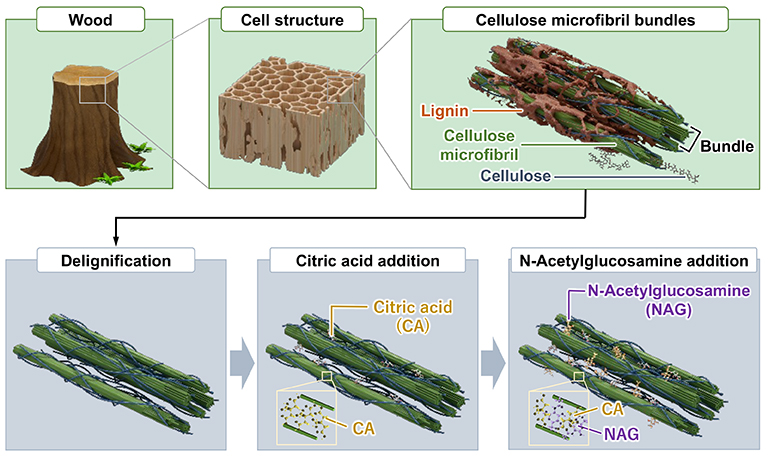
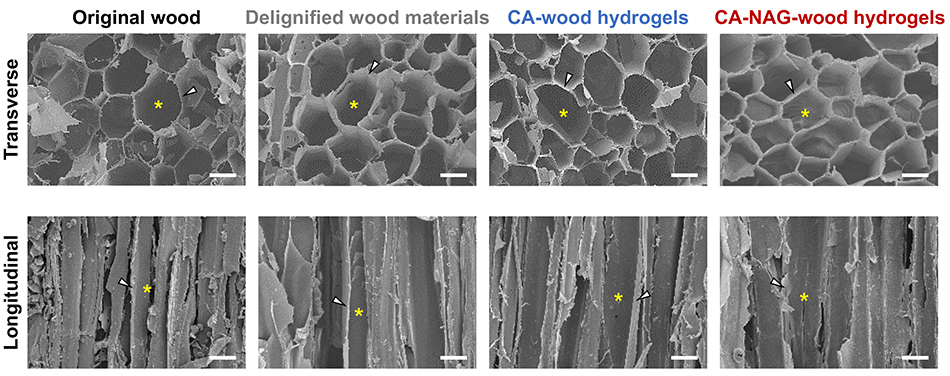
During the synthesis of wood-derived sponge hydrogel, the macroscopic structure of the wood remains intact, and the CA-NAG-wood hydrogel retains the original macroscopic structure of wood (Figure 2). The macropores, ranging from 40 to 80 μm, derived from cavities enclosed by wood cell walls, facilitate cell infiltration into the sponge hydrogel. Additionally, the struts derived from the wood cell walls contribute to the high strength of the sponge hydrogel.
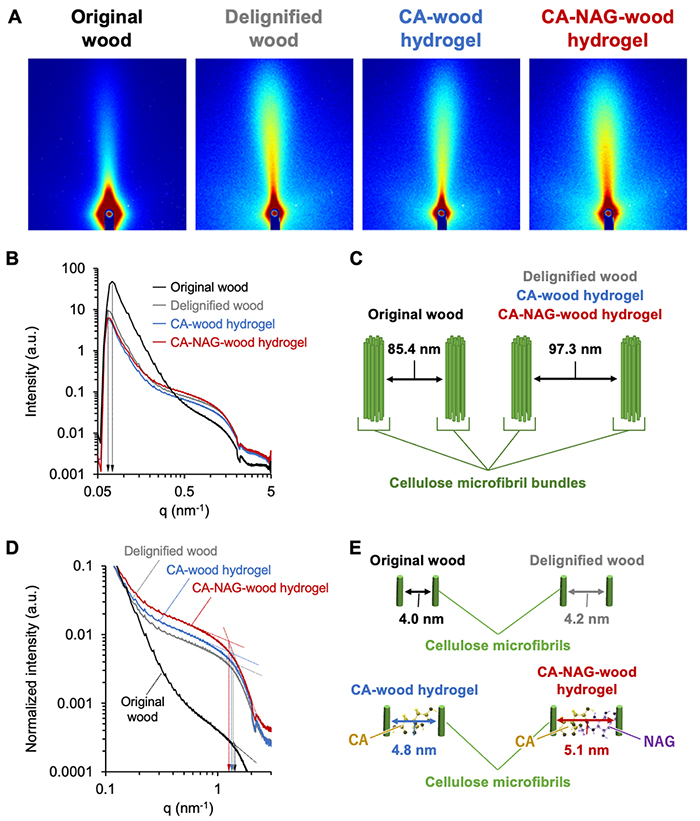
Evaluation of the crystalline structure of the wood-derived sponge hydrogel using wide-angle X-ray scattering (WAXS) revealed that the CA-NAG-wood hydrogel retains the cellulose crystalline structure of the original wood. Furthermore, as the synthetic process progresses, the proportion of the amorphous region increases, which imparts flexibility and elasticity to the sponge hydrogel. Additionally, small-angle X-ray scattering (SAXS) analysis of the wood-derived sponge hydrogel demonstrated that the distance between cellulose microfibrils expands as the synthetic process advances (Figure 3). This increased distance corresponds to the molecular sizes of CA and NAG, confirming that these molecules have been intercalated between the cellulose microfibrils.
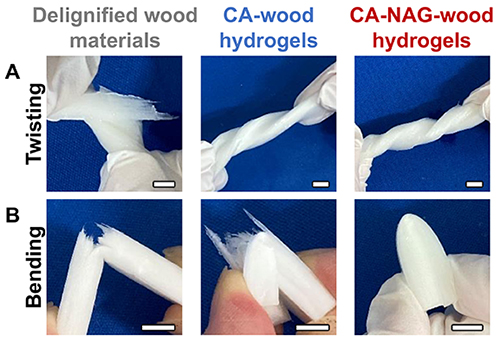
The physical and mechanical properties of the wood-derived sponge hydrogel undergo significant changes throughout the synthetic process. For example, delignified wood is susceptible to torsion stress and easily tears, whereas CA-wood and CA-NAG-wood hydrogels exhibit flexibility and can be twisted without tearing (Figure 4A). Additionally, delignified wood is brittle and prone to breakage when bent, while CA-wood hydrogel can be bent but is susceptible to tearing (Figure 4B). In contrast, CA-NAG-wood hydrogel remains flexible and can be bent without breaking or tearing. Moreover, properties such as water content, compressive modulus, compressive strength, and friction coefficient change significantly during synthesis, ultimately resulting in a CA-NAG-wood hydrogel with properties equivalent to those of cartilage. Furthermore, CA-NAG-wood hydrogel possesses cushioning properties, allowing it to recover its original shape even after 90% compression without damage.
No cytotoxicity was observed in human mesenchymal stem cells (hMSCs) cultured with the CA-NAG-wood hydrogel. Furthermore, after culturing hMSCs on the CA-NAG-wood hydrogel for two weeks, Alcian Blue (AB) staining indicated that this material induces chondrogenic differentiation. In contrast, this effect was not observed with collagen sponges, which are commonly used as scaffold materials for osteochondral defect repair.
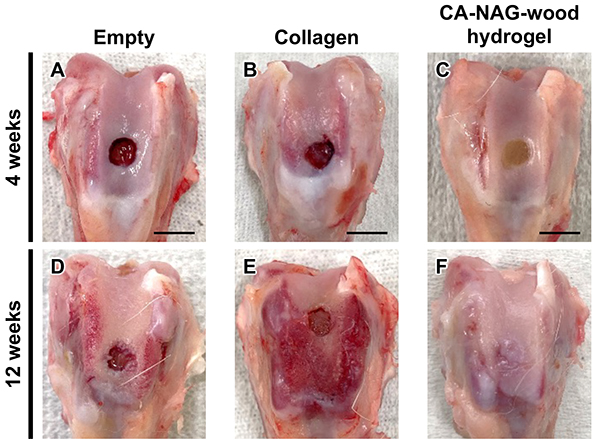
To evaluate cartilage regeneration ability, collagen sponge, delignified wood, CA-wood hydrogel, and CA-NAG-wood hydrogel were implanted into femoral subchondral bone defects in rabbits, dissected macroscopically, and subjected to histological analysis. Additionally, a comparison was made to an empty group (negative control) where no material was implanted. In this study, we present the main results of macroscopic dissection and histological images for the empty group, collagen group, and CA-NAG-wood hydrogel group. Furthermore, the handling of each material during implantation is an important factor for practical application. While the collagen sponge was fragile and disintegrated, the CA-NAG-wood hydrogel remained intact and was easily implanted.
At four weeks post-implantation, no tissue formation was observed in the subchondral bone defect areas of the empty group and collagen group (Figures 5A and 5B), whereas tissue formation was observed in the CA-NAG-wood hydrogel group (Figures 5C). At 12 weeks post-implantation, the defect area remained visible in the empty and collagen groups (Figures 5D and 5E), whereas in the CA-NAG-wood hydrogel group, the defect boundary became indistinct and tissue resembling the surrounding native tissue filled most of the defects (Figures 5F). Macroscopic morphological evaluation, based on a general grading scale, confirmed that the overall score was significantly higher in the CA-NAG-wood hydrogel group than in the other groups at both 4 and 12 weeks post-implantation (p < 0.01).
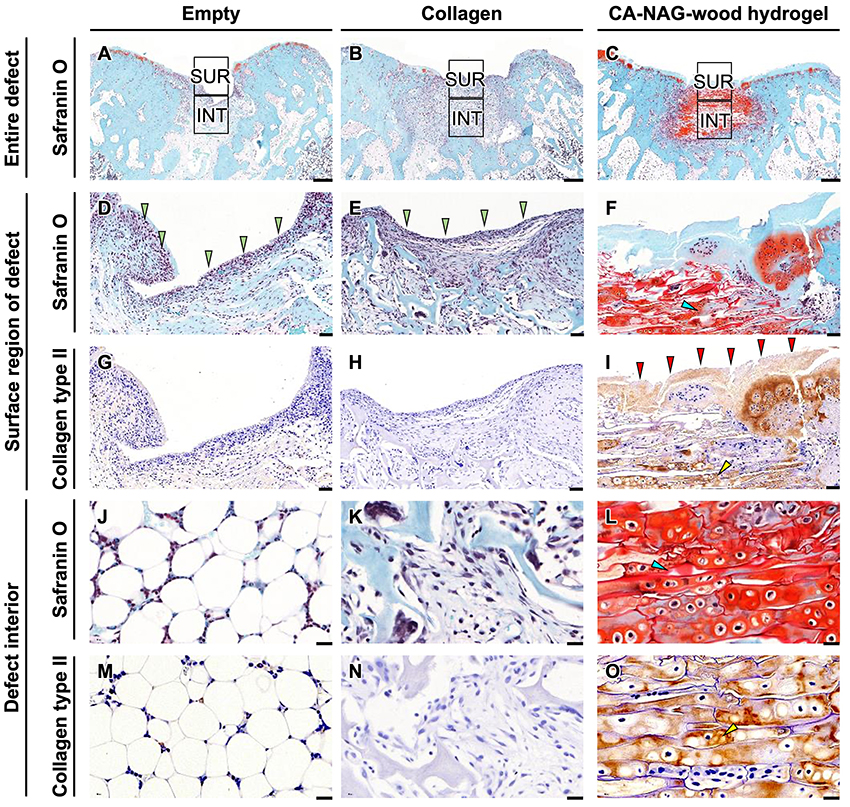
Due to space constraints, the corresponding figures are not presented. However, safranin O (SO) staining of tissue sections in the defect surface area at 4 weeks post-implantation revealed the formation of fibrous tissue in the empty and collagen groups, whereas in the CA-NAG-wood hydrogel group, a uniform and thick extracellular matrix (ECM) was formed throughout the region. Furthermore, immunostaining for type II collagen (COL2), a major component of cartilage tissue, failed to detect COL2-positive tissue in the empty and collagen groups. In contrast, in the CA-NAG-wood hydrogel group, the ECM formed in the defect surface region was immunostained for COL2, resembling cartilage tissue. In the subchondral defect area, SO-stained red-colored cartilage cells were not observed in the empty and collagen groups, but were partially visible in the CA-NAG-wood hydrogel group. Additionally, COL2-positive cells were not observed in the empty and collagen groups, whereas in the CA-NAG-wood hydrogel group, SO-stained red-colored cells were also COL2 positive, confirming the formation of cartilage cells.
At 12 weeks post-implantation, no SO-positive tissue was observed in the subchondral bone defect area of the empty and collagen groups (Figures 6A, 6B). However, in the CA-NAG-wood hydrogel group, SO-positive tissue was formed throughout the entire defect area (Figure 6C). The tissue formed in the defect surface region was fibrous in the empty (Figure 6D) and collagen groups (Figure 6E), whereas in the CA-NAG-wood hydrogel group, it was SO-positive ECM (Figure 6F). The fibrous tissue formed in the defect surface region was COL2-negative in the empty and collagen groups (Figure 6G, 6H), whereas in the CA-NAG-wood hydrogel group, the entire ECM was SO- and COL2-positive (Figure 6I). In the interior of the defect area, adipose tissue was present in the empty group (Figure 6J), and fibrous tissue was observed in the collagen group (Figure 6K). In contrast, in the CA-NAG-wood hydrogel group, SO-positive cells filled the macropores and were distributed throughout the hydrogel (Figure 6L). Moreover, no cells were COL2-positive in the empty and collagen groups (Figure 6M, 6N), whereas they were SO- and COL2-positive in the CA-NAG-wood hydrogel group (Figure 6O), confirming the formation of cartilage cells.
Analysis of the cartilage proportion in the defect area using an immunohistochemical technique at 4 weeks post-implantation confirmed that the value was significantly higher for the CA-NAG-wood hydrogel group (5.2 ± 1.8%) than for the empty group (0.0 ± 0.0%, p < 0.0001), collagen group (0.0 ± 0.0%, p < 0.0001), delignified wood group (1.0 ± 0.4%, p < 0.0001), and CA-wood hydrogel group (1.1 ± 0.3%, p < 0.0001). At 12 weeks post-implantation, the cartilage proportion was also significantly higher in the CA-NAG-wood hydrogel group (41.0 ± 8.8%) than in the empty group (0.0 ± 0.0%, p < 0.0001), collagen group (0.1 ± 0.1%, p < 0.0001), delignified wood group (0.4 ± 0.4%, p < 0.0001), and CA-wood hydrogel group (4.0 ± 3.6%, p < 0.0001). Furthermore, SO scoring evaluation revealed that the SO score of the CA-NAG-wood hydrogel group was significantly higher than that of the other groups at both 4 and 12 weeks post-implantation (p < 0.01).
By inserting CA and NAG between the cellulose microfibrils of wood, a flexible hydrogel with stiffness and strength comparable to the stiffness and strength of actual articular cartilage can be obtained. The CA-NAG-wood hydrogel exhibits shape recovery properties, returning to its original shape even after 90% compression, and possesses cushioning characteristics similar to natural cartilage. When implanted into the subchondral bone defect of a rabbit, the CA-NAG-wood hydrogel promotes the formation of cartilage extracellular matrix (ECM) on the defect surface at 4 weeks post-implantation, with the emergence of cartilage cells inside the defect area. By 12 weeks, cartilage cells are distributed throughout the defect site, and macroscopic observations confirm defect repair. These results were not achieved with collagen sponges. The favorable outcomes observed with the CA-NAG-wood hydrogel are likely due to the preservation of its wood-derived structural framework and its physicochemical and mechanical properties, which closely match those of cartilage.