
Masahiro Ogawa
Professor, Faculty of Agriculture, Kagawa University
Dr. Ogawa completed his master course at the Graduate School of Science and Technology, Sophia University in 1993 and obtained a doctoral degree in science in 1996. He worked as a JSPS overseas special researcher (dispatched to the University of British Columbia) for two years beginning in 1996 and then as a postdoctoral researcher at Louisiana State University and the University of Guelph. He began working for the Faculty of Agriculture, Kagawa University in 2004 and has been in his current position since 2009. His expertise lies in food science. His interests include food palatability and health functions.

Masashi Sato
Professor, Faculty of Agriculture, Kagawa University
Dr. Sato withdrew from the Graduate School of Agricultural Sciences, University of Tsukuba, after earning credits in a doctoral program and was appointed an assistant in the Faculty of Agriculture, Kagawa University in 1994. He obtained a doctoral degree in agricultural science in 1997 and has been in his current position since 2010. His research interests include the chemistry of bioactive natural substances. He is researching new bioactivities of rare sugars using C. elegans and other organisms.

Kazuya Akimitsu
Trustee and Vice-President of Kagawa University, Director of the International Institute of Rare Sugar Research and Education
Dr. Akimitsu completed his doctoral course at the Graduate School of Michigan State University in 1992 (Ph.D.). After working as a postdoctoral researcher at the MSU-DOE Plant Research Laboratory for 2 years, he began teaching at the Faculty of Agriculture, Kagawa University in 1994, specializing in rare sugar science and plant pathology. He was appointed the dean of the Faculty of Agriculture in 2021 and has been in his current position since 2023.
In the “Rare Sugars” series of articles of this journal, we first defined and classified rare sugars and then took an historical approach to describing their roles in nature under the title “What are rare sugars?”, tracking them from their position in the primitive earth to their current position. Next, we created the “Izumoring”, a systematic production strategy for rare sugars, tracked its evolution, and described the physicochemical properties of the crystalline structures of rare sugars and the features of the microbial enzymes used to produce rare sugars. We further proceeded to describe the development of applications of rare sugars, including the frontiers of research into medical applications, improved methods of cancer, diabetes, and obesity treatment. In this article, we provide an overview of the potential applications of the rare sugar D-allulose in food, the anti-aging effects of rare sugars, and the potential for agricultural applications.
Recently, there have been market launches of D-allulose-based processed foods. D-allulose, an isomer of D-fructose and D-glucose which are the major constitutes of isomerized sugars, suppresses blood glucose elevations and promotes fat combustion. As such, it is recognized as a functional food by the Consumer Affairs Agency in Japan. With these functionalities, some foods containing D-allulose in place of sucrose and isomerized sugars have been developed and marketed for health-conscious consumers. Reasons for adding sugar to food include providing energy supply, conferring sweet taste, increasing food preservability, improving texture, and promoting fermentation. Although sugars add a broad range of characteristics to food products, D-allulose exhibits properties different from those of sucrose when used in food.
Regarding energy, the energy conversion factor of D-allulose is extremely low at one-tenth that of sucrose and can be displayed as zero kilocalories on ingredient labels1. Accordingly, one of the features of D-allulose is that it will not increase the total caloric value, despite its identity as a sugar, even when added to food. Its sweetness is approximately 70% of that of sucrose, conferring a sense of refreshment and a sharp sweet taste.
Regarding texture, D-allulose exhibits properties that are distinct from those of sucrose in starch-rich foods such as Japanese confectionery and bread. As an example, its effect on rice cakes is described below. When used in place of sucrose, D-allulose softens gyuhi (mixture of glutinous rice flour and sugar) and glutinous rice flour dumplings2,3. This is because D-allulose and sucrose differ in their strength of the action on the starch in rice cakes. When glutinous rice flour, the major raw material for rice cakes, is heated in the presence of water, the starch molecules lose regularity and become glutinous. The gelatinization start temperature lowers as sucrose is replaced with D-allulose. The softening is attributable to a greater number of water molecules incorporated in the starch chain3-5. Another potent effect of D-allulose is the delaying of rice cake hardening during storage. Essentially, all of sugars delay rice cake hardening to some degree, D-allulose is more effective than sucrose and equally or even more effective than trehalose which is known to have a high anti-hardening effect2,3. This can be accounted for by the excellent anti-retrogradation effect of D-allulose on glutinous rice starch4. Interestingly, the D-allulose isomers D-fructose and D-glucose have only a low anti- retrogradation effect3,4. A similar investigation of starches other than those of glutinous rice showed that D-allulose had better anti-retrogradation effects than did other sugars on starches such as non-glutinous rice-derived starches, which have high amylopectin content ratios, but were only as effective as sucrose on potato-, wheat-, tapioca-, and corn-derived starches, which have relatively low amylopectin content ratios4. The greater anti-retrogradation effect of D-allulose over those of other sugars has been attributed to starch amylopectin6.
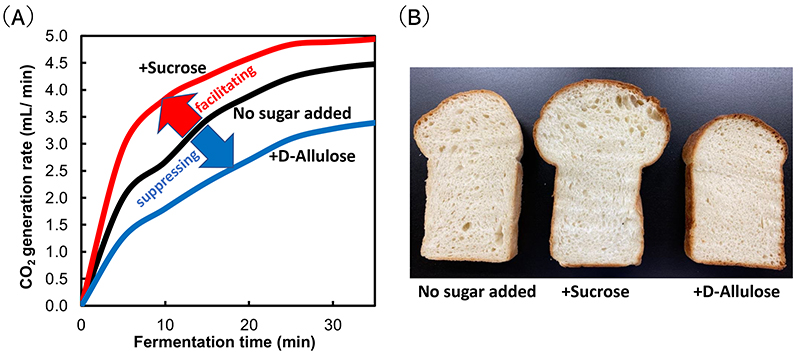
As for fermentation, D-allulose compared with sucrose sometimes behaves in a completely reverse manner. As an example, the fermentation of bread is described below. In the bread dough fermentation process, yeast performs alcohol fermentation using carbohydrates derived from wheat flour starch (D-glucose, etc.) as the substrate, producing ethanol (C2H5OH) and carbon dioxide (CO2). The CO2 from the yeast causes the bread dough to swell. When fermentation is performed with the addition of sucrose to bread dough, CO2 production increases, whereas the CO2 production decreases with the addition of D-allulose compared with the absence of the sugar7 (Figure 1A). For this reason, using D-allulose would produce hard bread as it hampers bread dough swelling7 (Figure 1B). The non-swelling of the dough is suggested to be because D-allulose competitively inhibits alcohol fermentation of the substrate by the baker’s yeast. Because the inhibition is competitive, adding sucrose to the dough concurrently with D-allulose cancels the inhibition of fermentation, making it possible to make soft bread8.
With its low caloric value and health benefits, as well as texture-improving effect, D-allulose is expected to find increasing applications as a substitute for sucrose and isomerized sugars in food. However, adaptive measures may be needed to overcome unwanted effects such as the inhibition of fermentation.
Calorie restriction (CR) is known to extend lifespan in laboratory animals and to delay aging-related changes and the progression of aging-related diseases9 and is recognized as one of the most effective anti-aging methods. However, because long-term CR is very difficult in actual practice, much attention has been given to the development of calorie restriction mimetics (CRMs), which have anti-aging effects similar to those of CR10,11. CRMs control metabolic regulation and genetic expression and exhibit anti-metabolic-syndrome and antitumor effects, resulting in a delay of senescence. Expecting that the zero-calorie carbohydrate D-allulose would become a CRM candidate, the present authors assessed its anti-aging effect using the nematode Caenorhabditis elegans, an experimental organism used in aging research.
The nematode C. elegans was cultured in a liquid medium containing D-allulose with Escherichia coli as food, and its lifespan was measured. When nematodes were treated with 10- and 25-mM D-allulose, their mean lifespan was extended by 12% and 7%, respectively, compared with control nematodes fed a D-allulose-free food12. In addition, when a nematode model of genetic CR with restricted food consumption (eat-2 mutant) was used to determine whether the nematode lifespan extension by D-allulose was due to a CR effect, no further extension of the lifespan of this mutant was demonstrated. Based on this result, the lifespan extension by D-allulose was attributed to an effect of CR12. At lifespan-extending concentrations, D-allulose elevated the AMP/ATP ratio and lowered intracellular energy levels in the treated nematodes (Sato, unpublished data).
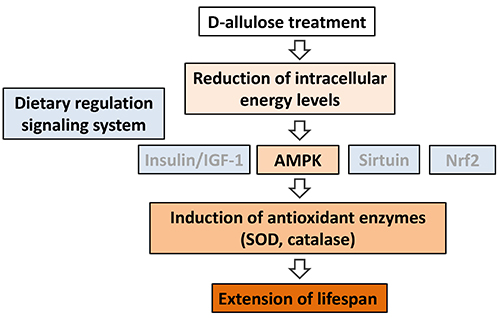
To identify what signaling system mediates the CR effect of D-allulose, a lifespan study was conducted using four mutants lacking a dietary regulation signal gene: daf-16 (a transcription factor downstream of the insulin/IGF-1 signal, forkhead box O [FOXO] homologue), aak-2 (AMPK homologue), sir-2.1 (sirtuin, silent information regulator-1 [SIRT1] homologue), and skn-1 (nuclear factor erythroid 2-related factor 2 [Nrf2] homologue). The results showed that D-allulose had a lifespan-extending effect in all mutants except the aak-2 mutant and that the effect depended on the AMPK signaling pathway12. D-allulose was also found to increase the mRNA expression and enzymatic activity of nematode antioxidant enzymes (superoxide dismutase [SOD], catalase)12. Based on these findings, the nematode lifespan extension by D-allulose is attributable to 1) intracellular energy level reductions, 2) AMPK signal activation, and 3) increased stress resistance due to enhanced expression of antioxidant enzyme (Figure 2).
On the other hand, the rare sugar D-allose, the C-3 epimer of D-glucose, extended the mean lifespan of nematodes by 24% when added at a concentration of 28 mM13. D-allose may be more effective in lifespan extension than D-allulose. A lifespan study with the same mutant as used in the D-allulose study showed that D-allose did not extend lifespan in the daf-16 and sir-2.1 mutants. This finding demonstrated that the lifespan extension mechanism of D-allose, unlike that of D-allulose, depended on the insulin/IGF-1 signal and sirtuin13.
It is an important goal of aging research to develop anti-aging substances that mimic the effect of calorie restrictions; as such, many CRM candidates are currently being studied. It is known, however, that the mechanism and signaling pathway for animal lifespan extension by different CRMs are complex and variable9-11. Furthermore, much remains unclear about the intracellular uptake of rare sugars and subsequent processes, such as their metabolism and enzymatic inhibition by the resulting metabolites. The mechanisms of these actions remain to be completely elucidated. The major advantage of rare sugars over other CRM candidates is their extremely low toxicity14,15, allowing for their commercialization as food. It is hoped that the effects of CRMs, especially the lifespan-extending effect, will be demonstrated in mammals. The discovery of any rare sugar having a sufficient CR effect is expected to make a significant contribution to the extension of human healthy lifespan.
The predominant monosaccharide on the Earth, D-glucose, is produced via photosynthesis in plants. Therefore, there is a profound association between plants and carbohydrates. In photosynthesis, chlorophyll pigment in plant leaves converts solar light energy into chemical energy and produces D-glucose from water and carbon dioxide. Photosynthesis takes place in two major stages: The first stage involves a photochemical reaction (or light reaction) in which chlorophylls absorb light energy and decompose leaf cellular water (H2O). Thus, oxygen (O2) is released, and adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide phosphate hydrogen (NADPH, reduced nicotinamide adenine dinucleotide) are produced. The next stage is the Calvin cycle (dark reaction or light-independent reaction), which uses the ATP and NADPH produced in the first stage, along with carbon dioxide (CO2) absorbed from the air to synthesize D-glucose (C6H12O6). Available as an energy source for all organisms, including plants, D-glucose is utilized for their growth and development. Plants, in particular, not only consume it as an energy source soon after its production, but also convert it to starch, cellulose, and other substances to be stored therein. Such stored sugars are utilized as energy sources by plants during non-photosynthetic periods and in dark environment. The carbohydrates stored as forms of starch and cellulose are further assimilated by other organisms into energy sources or effectively used as industrial resources such as paper and pulp.
Of the more than 400 thousand plant species that exist on Earth, all produce D-glucose via the aforementioned photosynthetic process, including some plants of the genus Itea, which are known to produce the rare sugars D-allulose and allitol16. Itea plants are deciduous shrubs or small trees, distributed mainly in South Asia and North America, with species of the same genus indigenous to the wetlands, riversides, and elsewhere in Japan. How do Itea plants produce D-allulose and allitol? This highly interesting research theme is under intensive investigation by many researchers, especially those at Kagawa University. A report suggests associations with photosynthesis, for example, although D-allulose content changes little in the dark, allitol content decreases markedly, and allitol is rapidly synthesized in bright light17. Furthermore, Enterobacter agglomerans strain 221e was shown to be capable of converting D-allulose into allitol18; a similar D-allulose-to-allitol conversion system may be present in Itea plants as well (Akimitsu et al., unpublished data).
Interesting results were reported from a study that investigated the effects of D-allulose and allitol on body fat accumulation in rats fed a high-fat diet prepared by mixing an ordinary feed with 5% dry Itea leaf powder19. In the same study, rats had free access to a mixed feed supplemented with 5% dry Itea leaf powder (hence containing 0.4% D-allulose and 0.6% allitol) or without for 8 weeks. Comparing the results from the two groups, it was found that the proportions of abdominal adipose tissue and total body fat were significantly lower in the group receiving the diet containing the dry Itea leaf powder than in the control group not receiving the same, suggesting an anti-obesity effect of the dry Itea leaf powder containing both D-allulose and allitol19. Hence, a further investigation was performed to determine the effects of concurrent intake of D-allulose and allitol, in which rats had free access to a diet containing 3% allitol, 3% D-allulose, or 3% allitol + 3% D-allulose for 8 weeks and were compared with a control group fed the same diet but lacking any of these sugars20. D-allulose significantly reduced final body weight, body weight gain, and the amounts of perirenal adipose tissue, mesenteric adipose tissue, and total abdominal adipose tissue, whereas allitol significantly reduced mesenteric adipose tissue weight20. However, D-allulose and allitol did not exhibit a combined effect on total abdominal fat, and allitol was suggested to be highly fermentable in the gut. D-allulose and allitol were suggested to have anti-obesity effects mediated by different mechanisms and to behave non-synergistically20. Besides their actions on animals, Itea virginica leaf components were found to inhibit the growth of lepidopteran insect larvae; allitol and D-allulose from leaf extracts inhibited the growth of Ephestia kuehniella Zeller (Pyralidae) larvae21. The EC50 values of allitol and D-allulose were reported to be 15.7 mg/g diet and 30.3 mg/g diet, respectively. D-allulose is already in food use and highly safe, and allitol is generally considered to be safe22, nonetheless, both were shown to be potentially useful as insecticidal agents21.
Prior to the above report on insect larva growth suppression by D-allulose21, approximately 50 rare sugars including D-allulose, D-allose, and D-tagatose, were studied to determine their potential agricultural use based on their actions on plants (e.g., induction of disease resistance and growth suppression) and their direct anti-plant pathogen effects23-29. Increasing evidence has shown that D-allulose or D-allose absorbed through plant roots or applied to the leaf surface transiently suppresses plant growth, induces the expression of a set of plant defense-related genes, and confers pest resistance via activation of the plant defense system against plant pathogens23-28. In addition, D-tagatose, unlike D-allulose and D-allose, does not affect the plants, but it is considered to act directly on fungi and thereby be potentially useful for pest control. Its mechanism of action was analyzed using the pathogen that causes “downy mildew”, a plant disease causing vast amounts of agricultural damage29. Phytopathogenic oomycetes, including downy mildew fungi, are rich in mannan and related substances produced via the D-mannose metabolic pathway; deficiencies and reductions in the components of these walls lead directly to the inhibition of fungal growth29. D-tagatose was demonstrated to inhibit the phosphorylation reaction of D-fructose to D-fructose 6-phosphate (F6P) by fructokinase in the first stage of the mannose metabolism pathway and to be converted to D-tagatose 6-phosphate (T6P) by the fructokinase29. The resulting T6P was shown to further inhibit phosphomannose isomerase in the same metabolic pathway, causing sequential decrease in the levels of the glycolytic pathway substrates D-glucose 6-phosphate and F6P, as well as D-mannose 6-phosphate, which is necessary for the biosynthesis of mannan and related products, in the various stages of the metabolic pathway, and inhibiting fungal wall formation, which is essential to the initiation of downy mildew fungus infection, conidiation, and other processes29.
The plant defense reaction induction and weed control activities mentioned above in the context of the effects of D-allulose and D-allose and the aforementioned direct bactericidal and bacteriostatic effects of D-tagatose on plant pathogens suggest their potential effectiveness in agricultural applications. These three rare sugars are very safe, with D-allulose and D-tagatose being globally marketed as food ingredients; rare sugars have the potential to serve as agricultural materials based on the idea that they act in a completely different manner from conventional agrochemicals.
While various features and functions of rare sugars were described in the past issues of the Rare Sugar Series, this paper describes some potential applications to the food, anti-aging, and agricultural fields and provides basic data supporting the development of applications for rare sugars. Although the development of applications for rare sugars was initially considered to be limited to the food area, carbohydrates have a history of use in a broad range of industrial fields. In this situation, we at Kagawa University, under the initiatives of the International Institute of Rare Sugar Research and Education, are investigating and developing new applications with the intention of replacing currently used carbohydrates with rare sugars in a broad range of industrial fields. In the food area, D-allulose is already being marketed in 16 countries. In other areas, including healthcare, agriculture, and industry, interesting research is proceeding at different rates, with some selected cases described in the present paper. It is hoped that commercial developments will continue to make the best use of rare sugar properties to facilitate the social implementation of rare sugar use. To this end, it is of paramount importance for not only university-based research alone, but also university-based research in close collaboration with industry, government, and academia, to make the best use of their own strengths. Fortunately, this collaboration is working very effectively for practical applications of rare sugars. In the coming issue, we will describe the history of industry-academia collaborations driving innovation in rare sugar technology to finalize the Rare Sugars Series in the present journal*.
(*Editorial note: This series temporarily concludes with its final episode here and will transition to irregular serialization thereafter.)
The authors express their gratitude to everyone who contributed to the preparation of this manuscript.