
Masato Nakagawa
Ph.D.
Junior Associate Professor, Center for iPS Cell Research and Application (CiRA), Kyoto University
First attracted to science by the study of biology in his high school (Tama Senior High School), Nakagawa entered Sophia University and then proceeded to the Nara Institute of Science and Technology (NAIST) in 1997, where and when he started his academic career. After working as a postdoctoral researcher at Nagoya University (2002-2004), he moved to Yamanaka’s laboratory at NAIST in 2004, then to the Institute for Frontier Medical Sciences, Kyoto University in 2005. In 2006, he witnessed the world’s first establishment of iPS cells. Thereafter, Nakagawa moved to the former CiRA, Kyoto University and he is now working for the current center. His research interests are elucidation of the mechanism of somatic cell reprogramming and the development of efficient methods for establishing and culturing iPS cells.

Shinya Yamanaka
M.D., Ph.D.
Director and Professor, Center for iPS Cell Research and Application (CiRA), Kyoto University
Senior Investigator at Gladstone Institutes
Representative Director of the CiRA Foundation
1987: M.D., Kobe University School of Medicine.
1993: Ph.D., Osaka City University Graduate School of Medicine.
1993: Postdoctoral Fellow, Gladstone Institutes in the United States.
1996: Research Associate, Osaka City University Medical School.
1999: Associate Professor, Nara Institute of Science and Technology Research and Education Center for Genetic Information.
2003: Professor, Nara Institute of Science and Technology Research and Education Center for Genetic Information.
2004: Professor, Institute for Frontier Medical Sciences, Kyoto University.
2012: Shared the Nobel Prize in Physiology or Medicine with John Bertrand Gurdon.
Since April 2010, Yamanaka has been in his current position of director and professor at CiRA. As the director of CiRA, he is working on promoting innovative studies, including elucidation of disease mechanism, drug discovery, and regenerative medicine, with the use of iPS cells, so as to realize medical applications of iPS cell technology.
About 15 years have elapsed since the birth of induced pluripotent stem (iPS) cells. Surprisingly, iPS cells have found some medical applications during that time. In this paper, we provide a historical overview of iPS cells, from their discovery to the present, and explore their future prospects.
In 1981, two groups, one in Europe and the other in the United States, reported that they succeeded in establishing mouse embryonic stem (ES) cells 1,2. ES cells are considered to be capable of differentiating into nearly all cell types in the body; in fact, individual mice can be produced from mouse ES cells. Hence, human ES cells, if created successfully, were expected to differentiate into a wide variety of cells, which may be used for regenerative medicine to restore functions in damaged tissues and organs via transplantation and the like. Upon establishing human ES cells for the first time in the world in 1998, researchers began studying their application to regenerative medicine 3. Knowledge and skills that had been acquired from previous studies with mouse ES cells were applied to human ES cells, enabling us to create a wide variety of cells.
In the context of regenerative medicine with stem cells, it is ideal to prepare self-derived stem cells, and to use them as the source to obtain cells for transplantation (Figure 1). As such, using ES cells in an attempt to realize the ideal form of regenerative medicine poses some challenges. First, the availability of source cells may be problematic. In Japan, human ES cells may not be created except from surplus embryos, which were originally prepared for infertility treatment by in vitro fertilization, but are to be discarded. (After developing for several days, fertilized eggs become embryos.) Therefore, it is not easy to create ES cells of self-origin. Another issue of concern is immune rejection. It is considered that transplanting ES cell-derived somatic cells, which are not self-originated, into a patient’s body will not result in successful transplantation because of immune rejection in most cases (Figure 1). In the actual medical practice of organ transplantation, immune rejection is prevented by immunosuppressants, which can often produce severe side effects. Another burden comes from the necessity for life-long use of immunosuppressants. The goal of ideal regenerative medicine cannot be achieved without obtaining the patient’s own stem cells to overcome these issues.
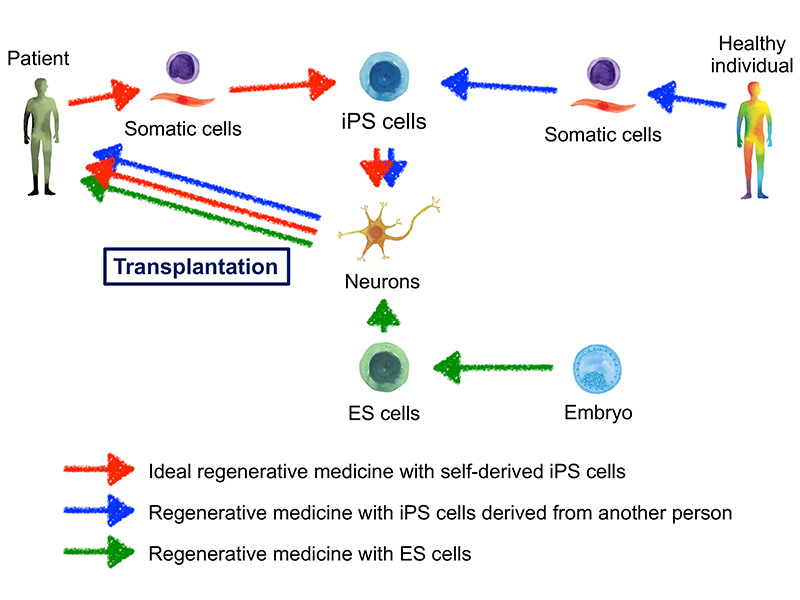
Judging from previous research at that time, it was thought that stem cells could be created from somatic cells. One example is a study conducted by Sir John Bertrand Gurdon on cell reprogramming via nuclear transplantation in the African clawed frog Xenopus laevis 4. In those days, it was commonly thought that only the fertilized egg possesses all the genetic information essential to the production of a living organism; however, Gurdon’s work showed that somatic cells likewise possess all the genetic information. He furthermore showed that a nucleus in the differentiated status could be reprogrammed and develop into an adult frog. Hence, his work demonstrated the potential of differentiated cells to be reprogrammed to acquire stem cell-like properties. Another study group showed that cells can change their nature when a transcription factor regulating gene expression is introduced. They reported that when a regulator of the muscle differentiation known as MyoD was introduced into fibroblasts, which are not muscle cells, they turned into myoblasts 5. That study demonstrated that somatic cells can be changed into other desired cells by introducing related factor(s), or gene(s). These two achievements suggested that certain cells collected from the body might be changed into stem cells by introducing genes which work actively in the stem cells.
The major issue was what factors needed to be introduced into somatic cells. Even if introduced randomly, several tens of thousands of genes would be difficult to examine. Just around that time, Dr. Yoshihide Hayashizaki and colleagues at RIKEN published a database of genes that are highly expressed in ES cells (http://fantom.gsc.riken.go.jp/), and we proceeded to select transgenes from the database. Eventually, we selected 24 genes, including one we identified which works to keep ES cells undifferentiated, as candidate reprogramming genes.
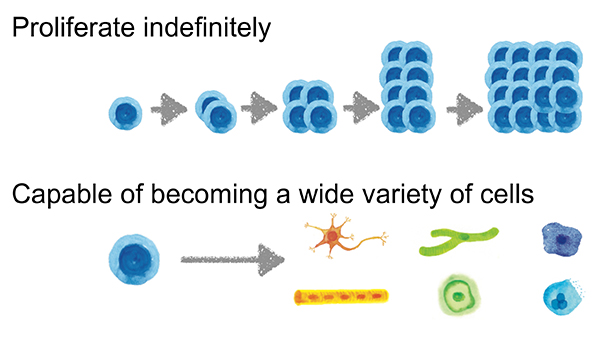
First, we introduced the 24 candidate reprogramming genes into mouse embryonic fibroblasts one by one. However, no ES-like cells were obtained. Then, we introduced all 24 genes into mouse embryonic fibroblasts at once and succeeded in obtaining ES-like cells, thus detecting the presence of reprogramming genes among the 24 candidates. To identify the critical genes, we introduced all combinations of 23 genes excluding one of the original 24 genes, and looked for the emergence of ES-like cells. As a result, we discovered that reprogramming can be achieved with no more than four genes, Oct3/4, Sox2, c-Myc and Klf4. In 2006, we reported for the first time in the world that stem cells can be produced by introducing the aforementioned four genes into mouse somatic cells, and named the stem cells induced pluripotent stem (iPS) cells 6. Various analyses showed that iPS cells, which are nearly ES cells, are capable of proliferating indefinitely and turning into a wide variety of somatic cells (Figure 2). Applying iPS cells to regenerative medicine makes it essential to create human iPS cells. As has recently been recognized, human and mouse ES cells have somewhat different properties; mouse ES cells are more undifferentiated than human ones. This means that cell culture conditions differ between the two types of ES cells. In 2005, our laboratory moved to the Institute for Frontier Medical Sciences (currently Institute for Frontier Life and Medical Sciences), Kyoto University, and continued our research there. At Kyoto University, Dr. Norio Nakatsuji and colleagues had already succeeded in the establishment and maintenance culture of human ES cells 7, and we announced for the first time in 2007 that we succeeded in creating human iPS cells by making the best use of their technology 8.
iPS cells and glycans are profoundly related to each other. This applies not only to iPS cells, but also to ES cells. In fact, to identify the target cells as human pluripotent stem cells (ES/iPS cells), glycan-recognizing antibodies, especially TRA-1-60 antibody, are often used as cell surface markers 9,10.
TRA-1-60 antibody recognizes a glycan modification, keratan sulfate, on the cell surface protein podocalyxin. It was originally reported to recognize a surface antigen of embryonic carcinoma (EC) cells, then it became to be used as a marker for human pluripotent stem cells as well. When human pluripotent stem cells get differentiated, the cells are no longer recognized by TRA-1-60 antibody.
As described above, TRA-1-60 antibody recognizes cancer cells. While human pluripotent stem cells are normal cells capable of self-replication, which are not immortalized due to genetic abnormalities, they can proliferate like cancer cells do. Therefore, TRA-1-60 antibody is unable to distinguish between human pluripotent stem cells and cancer cells. Recently, an antibody providing a solution to this problem, known as R-10G antibody, was reported by Dr. Toshisuke Kawasaki and colleagues at Ritsumeikan University 11. R-10G antibody, like TRA-1-60 antibody, recognizes a glycan modification, keratan sulfate, on podocalyxin, and has been shown to recognize keratan sulfate displaying a lower degree of sulfation. Because R-10G antibody recognizes human pluripotent stem cells but hardly recognizes human EC cells, it is expected to be more useful as a surface marker for human pluripotent stem cells. Much remains unknown, however, about the effects of glycan modifications of podocalyxin on the properties of human pluripotent stem cells; further advances in the relevant research fields are expected.
The world’s first clinical research project with the aim of applying iPS cells to cell transplantation took place in Kobe, Japan. A group led by Dr. Masayo Takahashi at RIKEN generated iPS cells from somatic cells of a patient with an eye disease known as age-related macular degeneration (AMD), and differentiated the iPS cells into retinal pigment epithelial cells. The iPS cell-derived retinal pigment epithelial cells were then transplanted into the patient’s eye in 2014. That operation represented the ideal regenerative medicine process with the patient’s own stem cells as described in the previous section of this paper (Figure 1). No adverse events have so far been reported from this study 12.
The same method used to culture human ES cells was used to culture human iPS cells and necessitated the use of feeder cells. Although much remains unknown about the detailed mechanism, co-culturing with feeder cells allows human pluripotent stem cells to grow stably, and to be cultured continuously.
However, iPS cells cultured using this method have posed problems that need to be resolved before their clinical application. Clinical application of the cells makes it essential to ensure that the culture media and reagents are safe for humans, which in turn can lead to the demonstration of the safety of the cells. The applicable guideline is the Standard for Biological Ingredients, and its administrative enforcement is under the jurisdiction of Japan’s Ministry of Health, Labor and Welfare. The culture method using feeder cells (feeder method) was found to require us to overcome many obstacles to meet the Standard for Biological Ingredients including the two main problems: culture medium and feeder cells. The medium contains bovine serum and swine enzyme, and these animal materials must be safe to humans as demonstrated by animal husbandry facility inspections and safety checks such as viral testing. In addition, since the feeder cells we used were an established cell line of mouse embryonic fibroblasts, they are required to meet the Standard for Biological Ingredients as with sera and other biological materials, which would add much cost and labor. The feeder cell quality must also be controlled. Although it was not absolutely impossible to address these issues, it was clear that a safer and simpler culture method would be required for medical application in the future. Hence, we started to develop a new clinically applicable method of culturing human iPS cells in the absence of feeder cells around 2010.
Around that time, one non-Japanese group reported that human pluripotent stem cells could be cultured stably for long periods by coating the culture dishes with the recombinant protein laminin-511 13. We also considered using laminin-511 in our project but gave up the idea because of the necessity to import it from outside Japan, its high cost, and for other reasons. While searching for another good coating agent, we stumbled upon the fact that Dr. Kiyotoshi Sekiguchi and colleagues at the Institute for Protein Research, Osaka University, was developing a method for producing an active fragment of laminin-511 (laminin-511E8), which only contains the portion essential for the binding activity, as a recombinant protein 14. We soon contacted him and commenced joint research. Thus, we found that laminin-511E8 could be used to establish, maintain and culture iPS cells without using feeder cells. In addition, Dr. Eihachiro Kawase and colleagues at the Institute for Frontier Medical Sciences (currently Institute for Frontier Life and Medical Sciences), Kyoto University, observed and reported similar findings 15. These facts demonstrated that laminin-511E8 is useful as a coating agent for culturing human pluripotent stem cells. Laminin-511E8 has been commercialized as iMatrix-511 jointly by Osaka University and Nippi, Inc., and is now available from MATRIXOME, Inc.
The next major issue concerned culture media. A medium contains a large number of ingredients, which are considered to act cooperatively in fostering cell growth. Commonly used media contain nutrient-rich fetal bovine serum (FBS). Although its composition is not fully defined, FBS has long been used for cell culture. Because animal-derived ingredients pose unknown risks such as infections, it is ideal to avoid their use for clinical application to the maximum possible extent. Collaborating with Ajinomoto Co., Inc., which possesses medium manufacture know-how, we began joint research to find the optimum medium for human pluripotent stem cells. Our efforts led to the successful development of StemFit®, a medium containing no animal-derived ingredients, which is free from materials subject to the Standard for Biological Ingredients. We thus succeeded in developing a feeder-free culture method, which combined StemFit® and the aforementioned iMatrix-511, to simply and very efficiently culture human pluripotent stem cells 16. This culture method is used in the iPS Cell Stock for Regenerative Medicine Project implemented at the CiRA Foundation (transferred from the Center for iPS Cell Research and Application (CiRA), Kyoto University, in 2020), as well as at many facilities working on clinical applications for iPS cells in Japan.
In Japan, clinical research projects and trials of iPS cells are ongoing; following the clinical research on AMD in Kobe using iPS cell-derived retinal pigment epithelial cells, clinical trials for Parkinson’s disease transplanting iPS cell-derived dopaminergic neuron progenitor cells 17 and for severe heart failure transplanting iPS cell-derived heart muscle cells 18 (at Kyoto University and Osaka University, respectively), and a clinical research project of iPS cell-derived platelet auto-transfusion for the treatment of thrombocytopenia (at Kyoto University) 19 are ongoing. More clinical research projects and trials with iPS cells are expected.
In Japan, human ES cells have already been finding clinical applications, and we will see further advances in regenerative medicine.
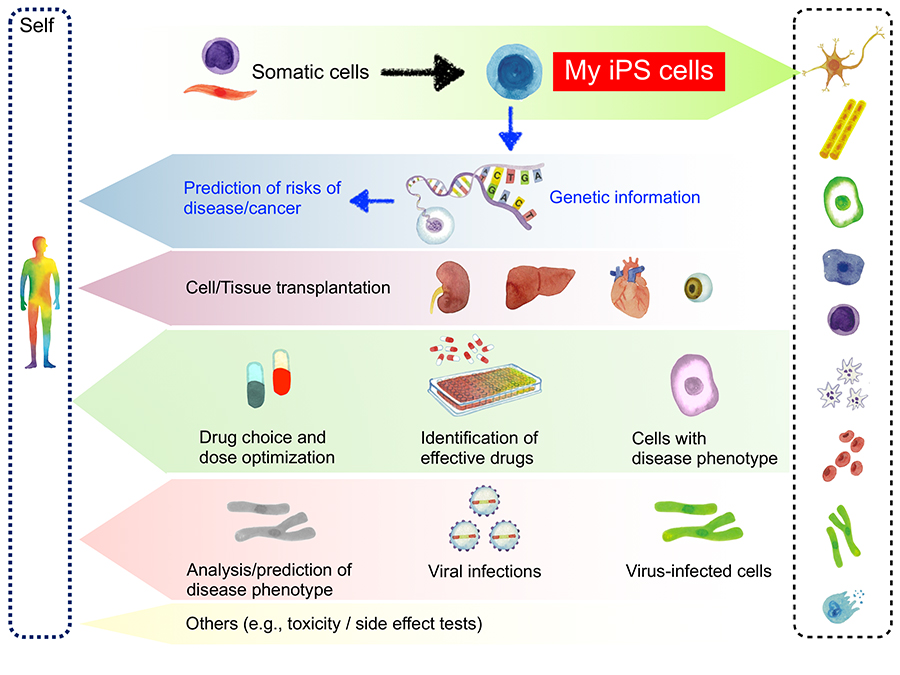
Many of the aforementioned cell transplantation is performed using iPS cells derived from a person other than the patient (Figure 1). This is because the preparation and quality assessment of iPS cells is time and cost intensive. However, the most attractive feature of iPS cells is that it offers the possibility of treatments and other approaches based on the patient’s own stem cells. In this paper, self-derived iPS cells are also referred to as “My iPS cells” (Figure 3). In Japan, several entities, including the CiRA Foundation, are working on building an array of services for contract manufacturing of My iPS cells, and some have already started accepting commercial orders. The forerunner is I Peace, Inc., which has begun providing production and banking services for personalized medical iPS cells. The news media reported that the services cost about 2 million JPY 20, an amount of money that may be paid personally. Similar services are likely to evolve and emerge in the near future.
An advantage of My iPS cells is that cells once lost because of disease or injury can be replenished by the patient’s own cells (Figure 3). Use of the patient’s own cells is expected to avoid immune rejection, thereby ensuring efficient cell transplantation therapy. It should be noted, however, that diseases and injuries in their acute stage cannot be treated with My iPS cells because some time is needed to generate the somatic cells from iPS cells. In addition, considerable cost is required to provide sufficient availability of the personalized cells used for transplant. However, the heart and other organs are likely to be generated from a wide variety of cells in the future, and My iPS cells may be used widely for transplantation.
Another use of My iPS cells is personalized medicine. Including the aforementioned transplantation strategy, personalized medicine as mentioned herein means the application of My iPS cells to drug discovery. Assume, for example, that liver cells are generated from the My iPS cells of a patient with liver disease and used to recapitulate his or her disease phenotype in a culture dish. The cells may be used for drug screening to identify the best medication for the patient, and even to optimize its dose and other factors (Figure 3). The human body is highly variable and individual, so that effectiveness of the same dose of the same drug may differ from one person to another; we believe that treatment can be optimized with the use of My iPS cells. Likewise, My iPS cells seem to be advantageous as they can be used to detect potential toxicities, side effects, and other aspects of drug action.
In addition, many types of cancer are known to develop from genetic abnormalities, and we consider that utilizing My iPS cells, which carry the patient’s own genes, could be used to predict canceration. Recent dramatic advances in genome informatics have enabled us to easily read the whole genome sequence, and the database on cancer-related genetic abnormalities is updated daily. Combined with such information, actual examination on culture dishes may be performed to determine whether somatic cells prepared from My iPS cells develop cancer, so as to predict canceration to some extent. In addition, by comparing the data on My iPS cells between individuals who have actually contracted cancer and those who have not, it may be possible to predict canceration due to single-nucleotide polymorphisms (SNPs), other than known genetic abnormalities (Figure 3). Furthermore, My iPS cells are likely to contribute to prediction-based cancer treatment.
We at CiRA are now generating iPS cells (CoV2-iPS cells) from somatic cells of patients who have become infected with the novel coronavirus, manifested some symptoms, and recovered from the illness. (The samples were collected after recovery and confirmed as negative by PCR.) Specifically, we are working to generate iPS cells from populations of severely, moderately, and mildly affected patients, respectively, and to distribute them as research materials to interested researchers. This project will enable us to prepare infection-related cells from CoV2-iPS cells and to analyze the mechanism of onset, and it is expected to lead to the elucidation of some of the mechanisms underlying progression to severe disease, which remain unclear despite efforts so far (Figure 3).
In addition, the potential use of My iPS cells is expected to increase with the compilation of findings on COVID-19. It may be feasible to get infection-related cells, which are generated from My iPS cells, infected with the novel coronavirus or similar so as to determine the risk of progression to severe disease. Furthermore, predicting an effective drug would enable us to treat the patient before he or she becomes severely affected.
As such, preemptive medicine, including phenotype predictions and drug effect assessments, with My iPS cells cannot emerge and evolve without using big data analytics. If we link a broad range of information to the My iPS cells services and construct a system for sharing it, we may be able to establish a groundbreaking medical system. For example, causal factors for disease that cannot easily be identified may be discovered by making the best use of a supercomputer, such as RIKEN’s Fugaku, which is now ranked No. 1 in the world, to process big data information and analyze the resulting data using artificial intelligence (AI). We expect the accuracy of this analytical system will increase to help realize better preemptive medicine as the application of My iPS cells spreads more and more.
Born to realize the ideal of regenerative medicine, iPS cells have already been steadily used to establish effective treatments in actual clinical settings. We hope the technology will evolve into next-generation medicine comprised of the new material My iPS cells, big data, and AI.