Hiroaki Tateno
My research aim is to develop new technologies for glycomics. Using the developed tools, I unravel previously unobservable life phenomena and develop practical technologies in medicine and industry.
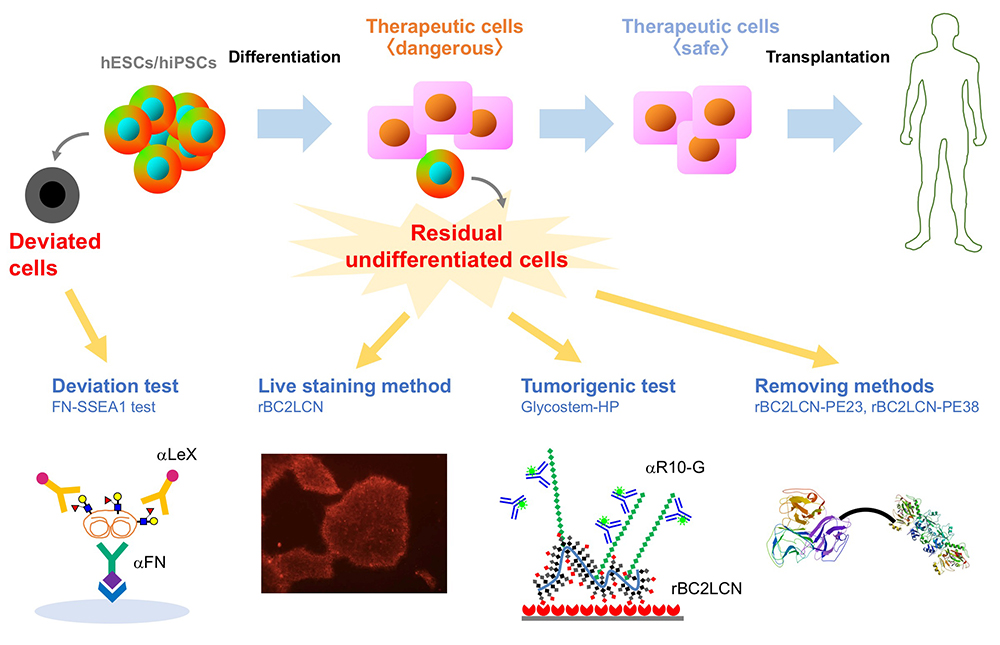
We performed comprehensive analysis of the cell surface glycomic signature of human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs). We clarified the characteristic feature of the glycome of hESCs/hiPSCs, and identified a lectin termed rBC2LCN that reacts with hESCs/hiPSCs. We then developed a technology to detect and eliminate hESCs/hiPSCs resided in cell therapy products (CTPs) using rBC2LCN. More recently, we developed a technique to detect deviated cells, cells that have deviated from the undifferentiated state of hiPSCs. Here, we would like to introduce glycan-based quality control technologies of the hESCs/hiPSCs that we’ve developed so far.
Human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs) with ability to proliferate indefinitely (self-renewal) and differentiate into all types of cells (pluripotency) are expected to be attractive cell sources for regenerative medicine. Currently, many clinical research projects are underway to effectuate the industrialization of regenerative medicine using hESCs/hiPSCs. In September 2014, the transplantation of retinal pigment epithelial cells prepared from autologous hiPSCs was carried out for the first time in the world for the purpose of treating age-related macular degeneration. Thereafter, cell therapy products (CTPs) prepared from hiPSCs were used for the treatment of various diseases such as Parkinson's disease, corneal epithelial stem cell exhaustion, severe heart failure, thrombocytopenia. As described above, while clinical application of hiPSCs is becoming practical, there is a major safety concern: tumorigenicity. In the “ Guideline on Ensuring the Quality and Safety of Drug Product, etc. Derived from Processing of Human (Autologous) iPS(-like) Cells ,” one of the most important requirements is to prevent the contamination of undifferentiated cells other than the target cells. It is desirable to eliminate the remaining undifferentiated cells other than the target cells at the intermediate product stage as much as possible. The tumorigenicity of residual undifferentiated cells is of great concern when the hiPSC-based CTPs are used for medical treatment. Therefore, in order to realize the industrialization of regenerative medicine using hESCs/hiPSCs, methods to detect and eliminate undifferentiated hESCs/hiPSCs remaining in CTPs are in demand.
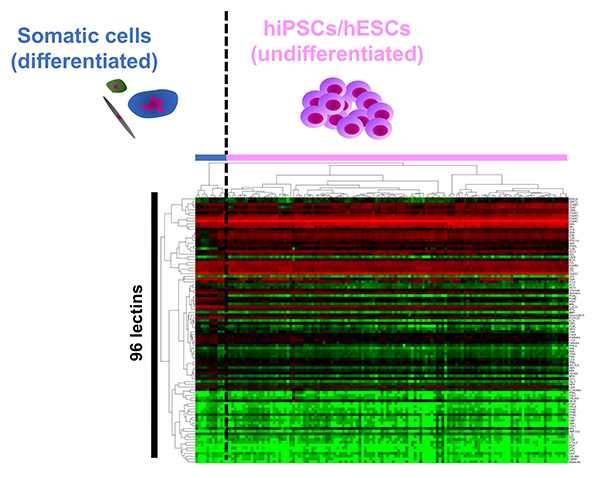
The cell surface is covered with glycans, which change in structure depending on the type and state of the cell. Therefore, the glycomic signature has been used as a cell surface marker for identification and selection of various cells. The commonly used surface markers of hESCs/hiPSCs such as SSEA-3/-4 and Tra-1-60/81 are glycans. However, the details of the glycan structure expressed in hESCs/hiPSCs are not fully known. Immediately after the generation of hiPSCs was reported in 2007, we used a glycan profiling technology called high-density lectin microarray to analyze 114 types of hiPSCs generated from various tissues such as human skin, fetal lung, amniotic membrane, endometrium, and placental artery. The glycan profile of the prepared hiPSCs was compared with the glycan profiles of parental human somatic cells and hESCs 1. 1) hiPSCs were classified into the same cluster with hESCs, and hESCs/hiPSCs were classified into clusters other than somatic cell clusters; 2) 114 hiPSCs were classified into one large cluster regardless of origin, and 3) somatic cells were classified into different clusters (Fig. 1). We demonstrated for the first time “glycan reprogramming” and hESCs/hiPSCs have a unique “glycomic signature”. So, what does the glycomic signature of hESCs/hiPSCs look like? Based on the obtained lectin microarray data and the results of gene expression analysis of glycosyltransferases, we identified three unique glycan epitopes expressed in hiPSCs: α2-6 sialic acid, type 1 lactosamine structure (Galβ1-3GlcNAc), and α1-2 fucose. In order to verify these results, N- and O-glycans on hiPSCs (201B7 strain) and human dermal fibroblasts (hFibs) were compared by HPLC and mass spectrometry2. As a result, 20 N-glycans and 7 O-glycans were identified from hFibs, and 37 N-glycans and 10 O-glycans were identified from hiPSCs. A clear difference was observed in glycan structure between the two types of cells, which is almost in agreement with the results of glycan profiling by lectin microarray. That is, in the N-glycans of hiPSCs, 1) all the sialic acid linkage patterns of N-glycans are α2-6 type; 2) type 1 lactosamine structure is detected, and 3) H antigen (Fucα1-2Gal) is detected. On the other hand, no significant change in the sialic acid linkage pattern was observed in the O-glycans, but the appearance of type 1 lactosamine and H antigen was remarkable.
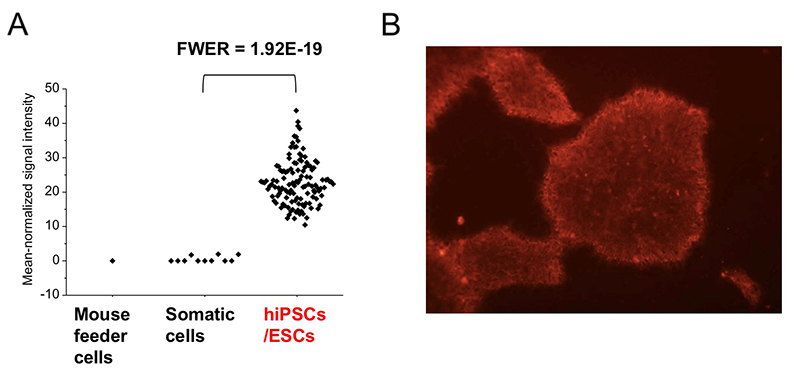
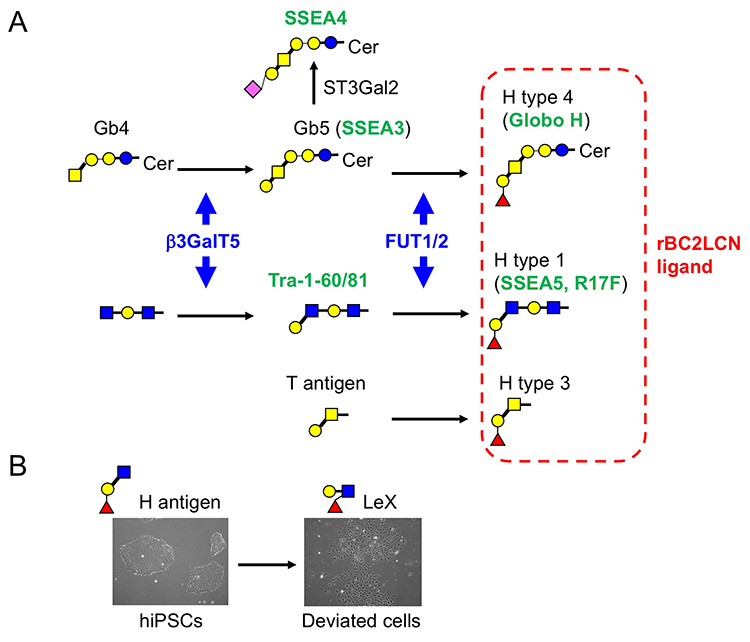
Among 96 lectins used in the lectin microarray, rBC2LCN, a recombinant form of the N-terminal domain of the lectin BC2L-C from the Gram-negative bacterium Burkholderia cenocepacia, was found to react with all undifferentiated hESC/hiPSCs, but never with differentiated somatic cells or mouse feeder cells (Fig. 2) 1,3. When the glycan-binding specificity of rBC2LCN was analyzed by glycan microarray and frontal affinity chromatography, the lectin showed binding specificity to Fucα1-2Galβ1-3GlcNAc/GalNAc containing the two (α1-2Fuc, Galβ1-3GlcNAc) of three characteristic glycan epitopes expressed in hESCs/hiPSCs described in the previous section. As a result of the above glycan structural analysis, core 2 O-type glycans having Fucα1-2Galβ1-3GalNAc were detected in hiPSCs 2. A search for a glycoprotein ligand of rBC2LCN revealed a sialomucin called podocalyxin as one of them 4. Practically, fluorescently-labeled rBC2LCN can be applied for live staining of various types of hESCs/hiPSCs just by adding fluorescently-labeled rBC2LCN to the cell culture supernatant (Fig. 2) 5. rBC2LCN is not toxic to hESCs/hiPSCs, even at concentrations as high as 100 µg/mL. Of course, the lectin can be used in flow cytometry as well as selective separation of hESCs/hiPSCs. The glycan epitope recognized by rBC2LCN is synthesized by glycosyltransferase genes, FUT1/2 and β3GalT5, whose expression is significantly increased in hESCs/hiPSCs (Fig. 3). In fact, these glycosyltransferases are also involved in the synthesis of known hiPSC/hESC markers such as SSEA-3/-4/-5, Tra-1-60/-81, and Globo H. Therefore, the glycan structure recognized by rBC2LCN is closely related to known hESC/hiPSC surface markers.
Furthermore, podocalyxin, a glycoprotein ligand of rBC2LCN expressed on the surface of hiPSCs (hereinafter, rBC2LCN-positive podocalyxin), was found to be secreted into the culture supernatant from various types of hESCs/hiPSCs 6. rBC2LCN-positive podocalyxin characteristic of hESCs/hiPSCs is specifically secreted from hESCs/hiPSCs, but not from normal somatic cells. That is, by examining rBC2LCN-positive podocalyxin in the culture supernatant, hESCs/hiPSCs can be detected using the culture supernatant without using the cells themselves. Therefore, a sandwich assay (GlycoStem-HP method) was constructed by using rBC2LCN as a capturing probe and R-10G antibody with specificity to low-sulfated keratan sulfate on podocalyxin as a detection probe 7. The GlycoStem-HP method is applicable to the detection of hESCs/hiPSCs cultured in various culture media. The lower limit of detection is 0.0006-0.03%. Furthermore, we verified whether the GlycoStem-HP method can be applied to actual transplanting cells derived from hiPSCs. This sandwich assay was applicable to the detection of hiPSCs mixed in with cardiomyocytes or human neural stem cells prepared from hiPSCs. This technology is now described in the “2019 Guideline for Detection of Undifferentiated Pluripotent Stem Cells/Transformed Cells, Tumorigenicity Test, and Genetic Stability Evaluation of Human Cell Processing Products”, and thus expected to be used in the regenerative medicine industry.
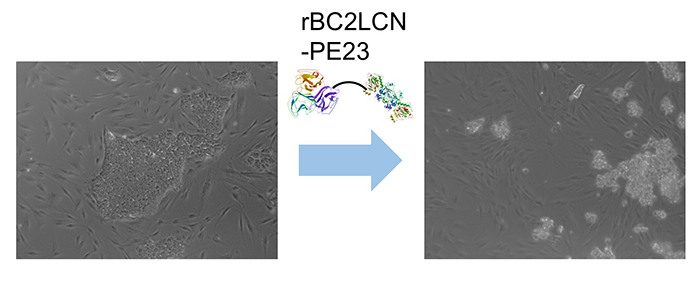
Interestingly, we found that rBC2LCN was internalized after binding to hESCs/hiPSCs 8. We hypothesized that rBC2LCN could be used as a drug carrier. Therefore, the catalytic domain (domain III, 23 kDa) of Pseudomonas aeruginosa exotoxin A, which inhibits protein synthesis in cytoplasm, was fused to the C-terminus of rBC2LCN (rBC2LCN-PE23). By 24 hours after adding rBC2LCN-PE23 to the culture medium, most of the hiPSCs lost their ability to adhere to the culture dish and floated in the culture medium (Fig. 4). This means that most hiPSCs have died. rBC2LCN-PE23 also showed the same effect on hESCs. On the other hand, it did not affect the survival or proliferation of hFibs and human mesenchymal stem cells (hMSCs). We also developed rBC2LCN-PE38 by fusing domain III as well as domain Ib and domain II of Pseudomonas aeruginosa exotoxin A to the C-terminus of rBC2LCN 9. rBC2LCN-PE38 had a 556-fold higher activity than rBC2LCN-PE23. When the killing effect of rBC2LCN-PE38 in a mixed cell population of hiPSCs and hiPSC-derived hepatocytes was examined, only hiPSCs were selectively killed and removed. hiPSCs remaining in hiPSC-derived CTPs could be selectively killed and removed by using rBC2LCN-PE38. More recently, we also developed a technique to remove hiPSCs from a population of differentiated cells using rBC2LCN-immobilized beads 10.
During cell culture, hiPSCs spontaneously undergo morphological changes from small cobble stone-like to large flattened shapes and lose pluripotent properties. Such cells are termed deviated cells, are altered from the undifferentiated state of hiPSCs, and express the early differentiation marker stage-specific embryonic antigen 1 (SSEA-1). During the transition from undifferentiated state to early differentiation state, the glycan shifts from H antigen (α1-2Fuc) to Lex (α1-3Fuc). We searched for SSEA-1-positive glycoproteins secreted into the culture supernatant from SSEA-1-positive deviated cells and found that fibronectin was one of the major carrier proteins of SSEA-111. Therefore, we developed an ELISA method termed the FN-SSEA-1 test to detect SSEA-1-positive fibronectin. Using this method, it is possible to nondestructively measure the number of deviated cells. Practical application of this technology to the quality control of hESCs/hiPSCs products is expected.
Here, I introduced the technology to detect and eliminate hESCs/hiPSCs using hESC/hiPSC-specific rBC2LCN. One of the biggest advantages of rBC2LCN is that the lectin is cost-effective and can be prepared in large quantities in Escherichia coli. In addition, the method of deviated cell detection was also described. Of course, cell surface glycans can be applied not only to hESCs/hiPSCs but also to the quality control of other CTPs. For example, although regenerative medicine using hMSCs has already been incorporated into medical practice, there is a lot-to-lot variation in the therapeutic effect of hMSCs, and therefore quality control technology of hMSCs is required. In this sense, we have also reported that α2-6 sialic acid is an indicator of the differentiation potential of human mesenchymal stem cells 12,13. We would be happy to contribute to the industrialization of regenerative medicine by developing various cell quality control technologies that utilize glycans.