
Tadasu Urashima
Obihiro University of Agriculture & Veterinary Medicine, Obihiro, Japan. Ph.D., Agriculture.
After graduation from Tohoku University (Doctor of Agriculture) in 1986, he started his professional career by studying milk oligosaccharides at Obihiro University. In 1991, he studied glycosyltransferase activity in lactating mammary glands of the tammar wallaby (marsupial) under Dr. Michael Messer in the Department of Biochemistry, the University of Sydney. Then he completed a comprehensive study of milk oligosaccharides of monotremes, marsupials, and several species of eutherians with Dr. Messer. He is interested in how the present milk components have been acquired during the evolution of mammals, especially how the acquisition of α-lactalbumin, a milk protein, has resulted in the appearance of milk oligosaccharides and lactose, and caused their biological significance to change during evolution. Since 2003, he has been a full professor at Obihiro University. At present he is president of the Japanese Society of Dairy Science and a board member of the Japanese Society of Carbohydrate Research (JSCR) and Japanese Consortium for Glycoscience and Glycotechnology (JCGG).

Jun Hirabayashi
Tokai National Higher Education and Research System, Nagoya University, Japan. Ph.D, Science.
After graduated from Tohoku University (Master of Science), he started his professional carrier at Teikyo University under Prof. Kenichi Kasai for the investigation of animal lectins. On the occasion of GlycoXV (Tokyo, 1999), he proposed the concept glycome; for this realization, he moved to National Institute of Advanced Industrial Science and Technology (AIST, Tsukuba) in 2002, and was involved in a series of national projects for glycan engineering, while he was a deputy director in Research Center for Medical Glycoscience (2006~), prime senior researcher of Research Center for Stem Cell Engineering (2012~). Now, he is a designated professor in Institute for Glyco-core Research (iGCORE), Tokai National Higher Education and Research System, Nagoya University, while being a vice president of the Japanese Society of Carbohydrate Research (JSCR) and Japanese Consortium for Glycoscience and Glycotechnology. (JCGG). He is also a visiting professor of Kagawa University (2003~) and Yokohama City University (2019~).
Mammalian milk or colostrum usually contains a variety of milk oligosaccharides in addition to lactose (Galβ1-4Glc) as the predominant saccharide. Almost all of them have a lactose unit at their reducing ends and also contain galactose (Gal), N-acetylglucosamine (GlcNAc), fucose (Fuc), N-acetyl or N-glycolylneuraminic acid (Neu5Ac and Neu5Gc, respectively), or N-acetylgalactosamine (GalNAc) residues. For example, human milk contains more than 250 varieties of oligosaccharides at a concentration of 12–13 g/L (see Note 1). Around 170 of the more than 250 chemical structures have been characterized to date.1 In this chapter of the series, the potential utilization of milk oligosaccharides is discussed as a unique research tool to solve the unanswered questions related to galectins.
Note 1: This concentration is equivalent to 14–16 mM, when 853 of lacto-N-fucopentaose I (LNFP-I), a major human milk oligosaccharide (HMO), is used as an average molecular weight. Most HMOs have structural motifs (glycotopes), to which galectins show significant affinity. For detailed features, see ref 1. Also, please note that the dissociation constants (Kds) for the interaction of galectin with HMOs are of the order of 10-4 M.
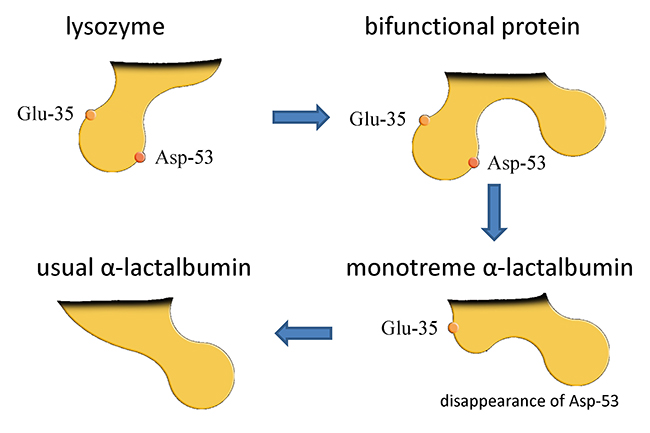
Although the definitive feature of mammals among the vertebrates is the secretion of milk, this character may not have been acquired at one specific time during the process of evolution. It is generally assumed that during the evolution of living mammals from synapsids via therapsids, cynodonts, and mammaliaforms, the present milk components were gradually acquired along with the acquisition of mammary glands and mammary cells. For example, it is hypothesized that casein, which is the most dominant protein in the milk of many species including the cow, evolved from an ancestral tooth enamel protein.2 α-Lactalbumin (αLA) is a milk protein that functions in the biosynthesis of lactose as a result of its association with β4galactosyltransferase 1 (β4gal-T1) within the Golgi apparatus of lactating mammary cells. It is considered to have evolved from c-type lysozyme, which catalyzes the hydrolysis of peptidoglycans in bacterial cell walls.
Lysozyme has two catalytic sites for this function. Even though the αLA of almost all species had lost these sites during the evolution from lysozyme, the αLAs of the platypus and echidna, two living monotremes, have retained one of these two sites. From this observation, it was hypothesized that a bifunctional protein for both lysozyme and αLA might have existed at one time (see Fig. 1). Although it can be assumed that the biosynthesis of lactose began as a result of the acquisition of αLA, the story is not so simple. Because milk oligosaccharides predominate over lactose in the milk of monotremes, which display some primitive features such as the absence of nipples and egg laying, it can be hypothesized that milk oligosaccharides were also dominant in the ancestral milk or milk-like secretions.3,4 It is known that milk oligosaccharides are synthesized by the actions of several glycosyltransferases which transfer monosaccharide residues from sugar nucleotides to lactose within the mammary cells. The low concentration of αLA in the milks of platypus and echidna suggests low expression of this protein in the lactating mammary glands of both species. The low expression of αLA would appear to be related to the low rate of lactose biosynthesis in these monotremes, and it can be hypothesized that a relatively high expression rate of mammary glycosyltransferases compared with that of αLA would produce a predominance of milk oligosaccharides relative to lactose in the milks of platypus and echidna (see Note 2).
Note 2: Several potential factors determine whether lactose predominates over the lactose-extended oligosaccharide structures in the milk of a particular species, or vice versa, and it is not easy to hypothesize a definitive key factor. However, we assume that the following changes might have occurred in relation to the acquisition of lactose during the evolution of mammals, namely the divergence of monotremes and therians (the ancestor of marsupials and eutherians) from a common ancestor, and then the divergence of marsupials and eutherians.
1) Enhancement of the affinity of αLA to β4Gal-T1, acquired as a result of mutation of αLA, to result namely in complex formation between αLA and β4Gal-T1.
2) Enhancement of the substrate specificity to glucose by formation of the complex of αLA with β4Gal-T1, to result namely in the decrease of Km value.
3) Increase in the expression level of αLA. Overall, the enhancement of the expression level of αLA would have increased lactose biosynthesis to about 1,000 times that in the ancestor in which αLA first appeared. In addition, it is thought that the energy acquirement mechanism of the suckling neonates had evolved by the acquisition of lactase (neutral β-galactosidase) in microvilli of the small intestinal epithelial cells; this must have contributed to the niche acquisition of the living mammalian subclasses. On the other hand, in milks of some eutherians such as humans, the concentration of milk oligosaccharides has also increased as well as that of lactose. It is speculated that the key factors determining the variety of acquired milk oligosaccharide structures may be as follows:
4) Enhancement of the expression of a few glycosyltransferases, which could have substrate specificity for lactose, such as i-β-1,3-N-acetylglucosaminyltransferase (iGnT) in lactating mammary epithelial cells.
5) Enhancement of the affinity of these enzymes to lactose or of the transfer activity of these enzymes.
6) Increase in the retention time of the lactose substrate within the Golgi apparatus.
7) Increase in the supply of donor substrates such as UDP-GlcNAc.
8) Enhancement of the function of potential transporters.
To test these possibilities, which are merely speculative at the moment, future study is necessary.
It seems probable that in the ancestors of eutherians the expression levels of αLA increased during their evolution, and thus the ratio of lactose to milk oligosaccharides also increased to the extent that lactose became the predominant saccharide in most eutherian species. In suckling eutherian neonates, the milk lactose is hydrolyzed to glucose and galactose by the action of lactase (neutral β-galactosidase), an enzyme which is located on the brush border of small intestinal epithelial cells. These monosaccharides are then transported into the cells by active transport, whereupon glucose enters the circulation while galactose is converted to glucose in the liver to be used for energy production. Thus, as a result of the acquisition of small intestinal lactase activity, the utilization of lactose as an immediate energy source became possible.
In contrast, the small intestines of monotremes and several species of marsupials have been shown to lack this brush border lactase as well as other brush border glycosidases, such as fucosidase and sialidase, which would be required for the digestion of milk oligosaccharides. For example, the milk oligosaccharides of the echidna, a monotreme, are mainly fucosyl and sialyllactoses; their digestion would most probably require the initial removal of the fucose and sialyl residues by specific glycosidases. In the tammar wallaby, a marsupial, the most prominent milk oligosaccharides are β1-3 linked galactosyl structures whose digestion would require at least one specific β-galactosidase. In view of the absence of brush border lactase it is likely that in the neonates of these species intact milk oligosaccharides could enter the small intestinal cells by pinocytosis or endocytosis and be transported to lysosomes, probably by a mechanism similar to autophagy, to be hydrolyzed to monosaccharides by lysosomal glycosidases. This hypothetical mechanism would solve the problem of how the milk oligosaccharides of monotremes and marsupials can be utilized as energy sources by their suckling neonates.
An alternative mechanism is the one generally accepted for the neonates of humans and other eutherian species, in which most of the milk oligosaccharides cannot be digested within the small intestine, again because of the absence of the relevant brush border glycosidases such as fucosidases and sialidases. The major part of human milk oligosaccharides (HMOs) reaches the colon where they can be metabolized by beneficial microorganisms, such as bifidobacteria, that inhabit the colons of breast-fed infants. The end-products are mostly organic acids which can be absorbed to provide energy. The biochemical pathways by which HMOs are metabolized by bifidobacteria have recently been largely clarified.5 Bifidobacterium bifidum has an extracellular digestion system for HMOs with a pathway that is specific for type Ⅰ HMOs that have a Galβ1-3GlcNAc (LNB) unit, whereas Bifidobacterium longum subsp. infantis has another HMO digestion pathway involving HMO-related gene cluster 1, which includes genes for transporting HMOs into the cell’s interior and also of several intracellular exoglycosidases. Although β-galactosidase encoded by an HMOs cluster 1 gene does not have an affinity for LNB, it has been found that another β-galactosidase, which is encoded by a gene located outside this cluster, can hydrolyze LNB.6 In human milk, the type Ⅰ oligosaccharides predominate over the type Ⅱ; that feature is specific to humans and not other mammals including non-human primates. From this fact one can hypothesize that bifidobacteria might have been evolved to acquire the metabolic ability to utilize type Ⅰ HMOs for their growth. This means that there has been a coevolution of human beings with colonic bifidobacteria, as the milk oligosaccharides concentration is much higher in humans than in many other mammalian species.7 In addition, it has been shown that HMOs can be utilized by some species of colonic bacteroides and eubacteria, suggesting that the metabolism of HMOs could be related to the composition of the colonic flora, even after weaning.8
HMOs may also act as anti-infection agents against harmful microorganisms and as immune modulators and agents that strengthen the colonic barrier function. The hypothetical anti-adhesive mechanism by which HMOs may protect against infection by pathogenic microorganisms, such as rotavirus, is based on the observation that the number of virus particles attached to epithelial cells in the presence of HMOs decreases relative to that of controls in the absence of HMOs. However, the mechanism may not be straightforward, because the number of virus particles attached to the cells increased in other experiments in which a different rotavirus strain was utilized.9 It is thought that the level of virus-specific immune IgG would increase in the presence of HMOs, and the inhibition of virus infection might be achieved by immune enhancement induced by the addition of HMOs.9,10
By changing the expression of the glycogenes in epithelial cells, the adhesion of HMOs to epithelial cell surfaces may be a key to the modification of the structures of surface glycoconjugates or mucins.11 Modification of these carbohydrate moieties could strengthen the barrier function, which may help to prevent necrotizing enterocolitis, a serious disease affecting immature infants in many countries.12 Although the detailed mechanism by which HMOs affect functions such as anti-infection, immune modulation, colonic barrier strengthening, etc., has not yet been completely clarified, it is assumed that the interaction of HMOs with some endogenous lectins is involved. In fact, Noll et al. showed in experiments using comprehensive glycan microarray, that galectins13 and dendritic cell specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN)14 bind with some affinity to a panel of HMOs. The binding specificity of galectin will be described in the next paragraph.
To date, as many as 170 HMOs have been characterized and will be utilized as useful tools to investigate galectin functions and their glycoconjugate ligand structures relevant to infection and immune reactions.1 As described, milk oligosaccharides are biosynthesized by the actions of several glycosyltransferases using lactose as a substrate. These are supposed to catalyze the biosynthesis of relevant carbohydrate structures, such as extensions, branches, and terminal modifications of glycoproteins and glycolipids; notably, the chemical structures of glycoproteins resemble those of glycolipids. The expected biosynthetic pathways of 19 core structures of HMOs other than lacto-N-novopentaose I [Galβ1-3(Galβ1-4GlcNAcβ1-6) Galβ1-4Glc] are shown in Fig. 2.1 As the core structures contain type II LacNAc (Galβ1-4GlcNAc) and type I LacNAc (Galβ1-3GlcNAc), the addition of fucose to these units form Lewis a [Galβ1-3(Fucα1-4)GlcNAc], b (Fucα1-2Galβ1-3[Fucα1-4]GlcNAc), or x (Galβ1-4[Fucα1-3]GlcNAc), which are common units in the glycoconjugates. Analysis of the interactions of HMOs with galectins may provide useful clues to the development of anti-infection or immune modulating drugs. For example, consensus rules for galectin recognition have been established: Galectins bind to both type Ⅰ and type Ⅱ LacNAc units, but not to these β-galactosides, which have modifications at the C4 or C6 position. Also, they do not bind to type Ⅰ or type Ⅱ LacNAc, where the GlcNAc units are substituted by α-fucose at the C3 or C4 position, respectively, namely, Lewis a or x units (disaccharide units shown in gray in Fig. 3). On the other hand, some galectins will show much stronger affinity for β-galactosides, which have undergone substitution at the C3 position depending on the modified groups. These observations are important with respect to the exploration of the functionalities of galectins, e.g., in development of galectin inhibiting drugs.13,14 The consensus rules thus described clearly predict that some milk oligosaccharides will never bind to galectins or some galectins may bind extraordinarily and also strongly to a particular set of structures, depending on the types of modifications (e.g., fucosylation and sialylation) of type Ⅰ/Ⅱ LacNAc (see Fig. 3).15,16 Hence, HMOs will be valuable research tools to explore the functionality of galectins developed as drug candidates. Further assessment of the precise interactions between galectins and available HMOs, whose chemical structures are defined, will generate more advanced designs for the development of drugs.17
As it has been elucidated that a small quantity of HMOs is absorbed and enters the circulation to be excreted in urine,18 it is assumed that HMOs may interact with galectin or selectin during circulation. Is there any possibility that the human characteristic type Ⅰ HMOs affect cellular homeostasis, which may be advantageous for neonatal health, in relation to the specificity of endogenous galectins, which prefer type Ⅰ to type Ⅱ LacNAc? Recently it has been reported that the release of galectin from small intestinal epithelium is stimulated by exposure to LNFP-I, a major HMO, together with CpG DNA, which imitates the bacterial product.19 As many HMOs with affinity to galectins exist in human milk/colostrum, and the effective concentration to reach to the gastrointestinal epithelium should be several to dozens of millimolar (mM), some HMOs must interact with galectins expressed in this epithelium when the infants suckle their mothers’ milk. Of note, galectins do not bind to all HMOs equally and with the same affinity, which largely depends on modification with sialic acid or fucose, their linkage positions, and preference for LacNAc structural types. These findings may provide hints to guide the exploration of the function of galectins expressed in the gastrointestinal epithelium in suckling infants.
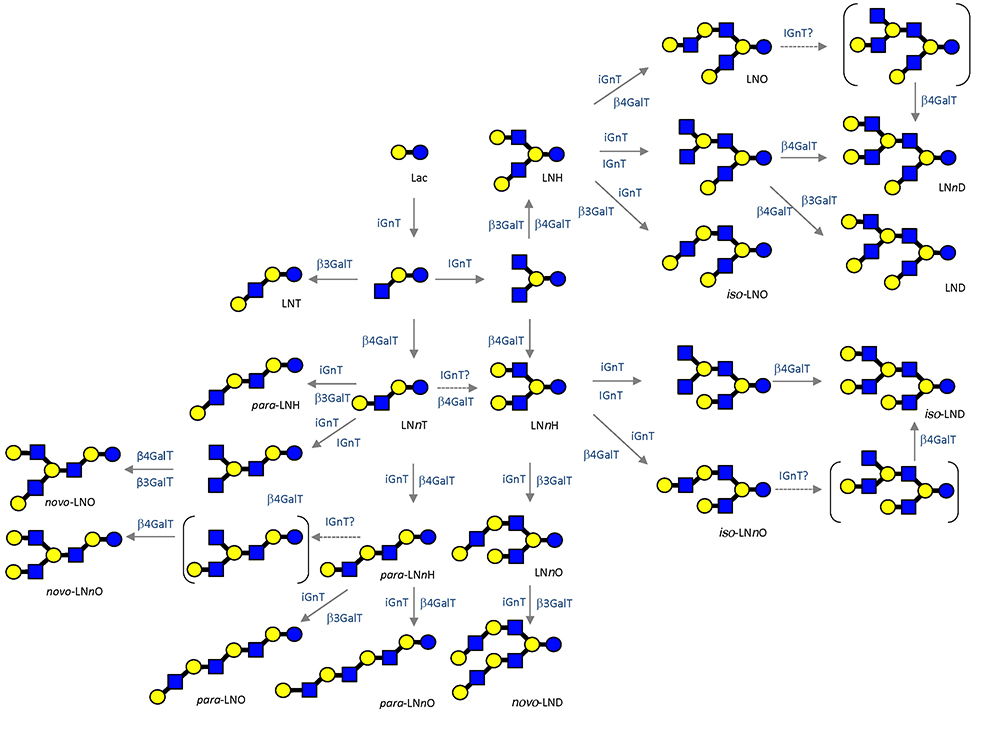
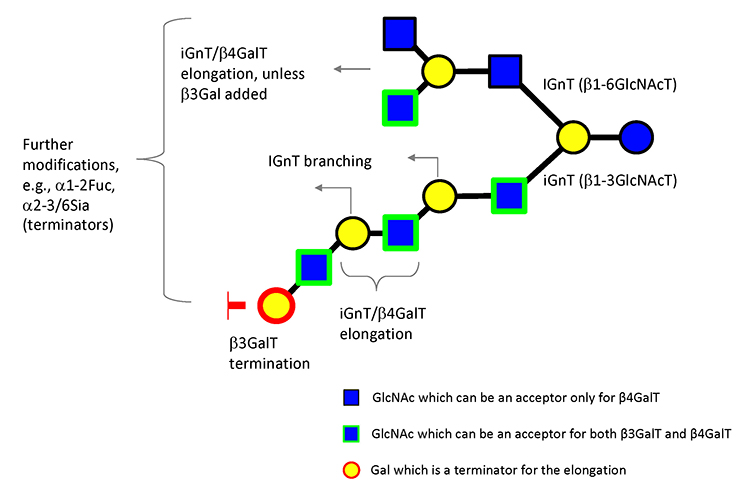
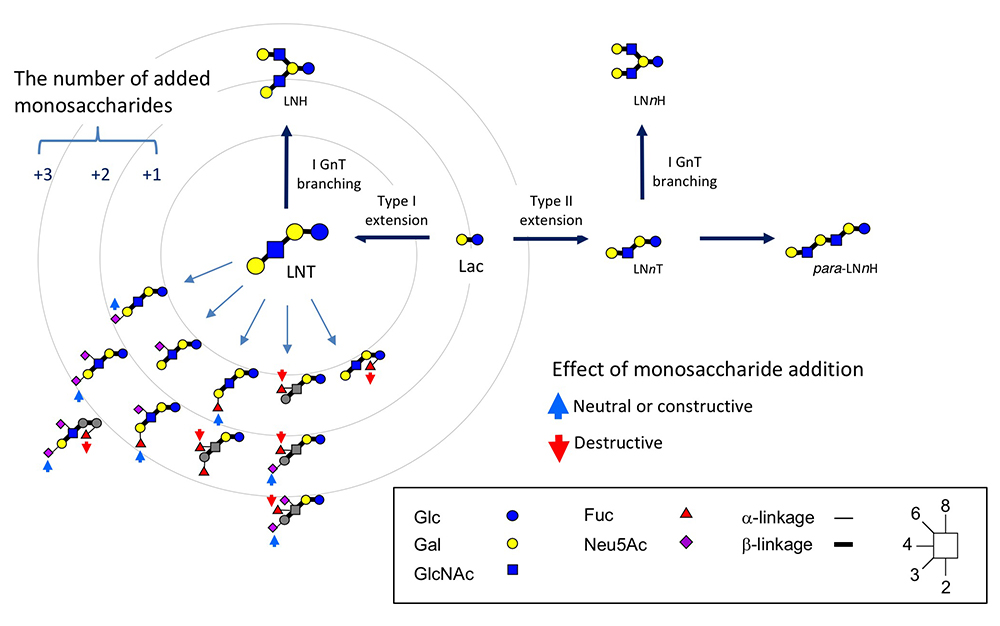
To explore the relationship of milk oligosaccharides to the formation of colonic microflora and also to immune modulation, it is hoped that animal (e.g., rat and mouse) studies will be performed. Even though the variety of milk oligosaccharides in rats is very limited, as this milk contains almost only Neu5Acα2-3Galβ1-4Glc (3’-SL) and Neu5Acα2-6Galβ1-4Glc (6’-SL),20 both the concentrations of these sialyllactoses and their ratio to lactose are very high. In fact, the concentration is estimated to be 16–14 g/L (i.e., 26–22 mM) at 4–8 days postpartum and this ratio to lactose is 0.61–0.51.21 As described in the previous section, the galectins usually bind to 3’-SL but not to 6’-SL. In addition, the binding ability of galectin to 3’-SL is at a sub mM level (i.e., that of galectin 3 is Kd=0.56 mM), and it is speculated that some galectins, which are expressed in the epithelium of the gastrointestinal tract, might have a chance to interact with milk oligosaccharides, such as 3’-SL, to trigger some biological functions. As strong neuraminidase activity, possibly of lysosomal origin, has been detected in the small intestinal tissue in suckling rats, it is speculated that a part of sialyllactose in milk might be imported into the intestinal cell interior by pinocytosis or endocytosis to be digested within lysosomes.22 Although it is unknown how much sialyllactose is absorbed in the small intestine, the determination of the rate of the absorption (small intestine) to reach (colon) levels should be important to explore the interaction between sialyllactose and galectins.
On the other hand, the milk of paca, one of the rodent species, shows a more complicated oligosaccharides profile (unpublished results, Urashima T et al.): it is speculated that most of the oligosaccharides other than sialyllactose should have been lost in rat milk in the course of breeding animals for experimental use. The group of Prof. Osawa, Kobe University, found that Enterococcus gallinarum, which had been isolated from the feces of suckling rats, could hydrolyze 3’-SL to N-acetylneuraminic acid and lactose; it is concluded that this species should be a key bacterium in the formation of the colonic microbiome influenced by metabolism of milk oligosaccharides.20 In the case of humans, bifidobacterial dominant microflora are formed under conditions of HMOs metabolism by bifidobacteria. This observation is important because it suggests that humans have a specific mechanism of bifidus flora formation based on utilization of unique milk oligosaccharides. By the way, it has been shown that many galectins are secreted by the epithelial cells. For example, galectin-3 and -4 are mainly secreted from the apical and basolateral surfaces of epithelial cells, respectively.23 It is hoped that the role of interaction of these galectins and sialyllactose in biological functions such as gastrointestinal immune modulation will be explored in future in vivo studies.
A diversity of milk oligosaccharide structures have been observed in mammalian species. Although HMOs do not have blood group A (GalNAcα1-3[Fucα1-2]Gal) or B (Galα1-3[Fucα1-2]Gal) antigens or α-Gal epitope (Galα1-3Gal) units, these have been found in the milks of other species, including bears.24 Linear β1-3 galactan-like oligosaccharides have been found in the milk of several marsupial species24 and these unique oligosaccharides are also available for studies on their interaction with several galectins. As these galactans resemble the epitope structures on the cell surface of Laishmania major, a protozoan that causes a serious infective disease especially in Central Asia, they were utilized in a study exploring their interaction with several galectins. The result showed an affinity with galectin-3 and -9, suggesting that this protozoan could use these galectins to invade the host.25 Based on this finding, one may be able to develop an anti-infection drug against L. major (i.e., leishmaniasis).
In summary, it can be concluded that lactose and milk oligosaccharides have been acquired during the evolution of the mammary glands of mammals. As a result, a diversity of milk oligosaccharides has also evolved, depending on the mammalian species, and their interactions with various endogenous lectins including galectins can be used to find new functions. Exploring the overall evolutionary development and potential functions of these milk oligosaccharides could provide us with critical information to develop a new concept of glycan drugs for a new industry.