
Tadasu Urashima
Obihiro University of Agriculture & Veterinary Medicine, Obihiro, Japan. Ph.D., Agriculture.
After graduation from Tohoku University (Doctor of Agriculture) in 1986, he started his professional career by studying milk oligosaccharides at Obihiro University. In 1991, he studied glycosyltransferase activity in lactating mammary glands of the tammar wallaby (marsupial) under Dr. Michael Messer in the Department of Biochemistry, the University of Sydney. Then he completed a comprehensive study of milk oligosaccharides of monotremes, marsupials, and several species of eutherians with Dr. Messer. He is interested in how the present milk components have been acquired during the evolution of mammals, especially how the acquisition of α-lactalbumin, a milk protein, has resulted in the appearance of milk oligosaccharides and lactose, and caused their biological significance to change during evolution. Since 2003, he has been a full professor at Obihiro University. At present he is president of the Japanese Society of Dairy Science and a board member of the Japanese Society of Carbohydrate Research (JSCR) and Japanese Consortium for Glycoscience and Glycotechnology (JCGG).

Sachiko Sato
Research Centre for Infectious Diseases, Faculty of Medicine, Laval University, Quebec City, Canada. Ph.D, Pharmaceutical science
Sachiko Sato graduated from Faculty of Pharmaceutical Science, Chiba University. She joined as postgraduate student in the laboratory of Dr. Akira Kobata, the Institute of Medical Science, the University of Tokyo, Japan in 1987. She also worked in the laboratory of Dr. R. Colin Hughes, MRC: National Institute for Medical Research in London, UK, where she first encountered a cytosolic mammalian lectin, now called galectin-3. She obtained her Ph. D. from the University of Tokyo in 1994. As postdoctoral fellow in the laboratory of Dr. Ron Kopito, Stanford University, she was involved in the work on cystic fibrosis. She became principal investigator of the laboratory of glycobiology in Research Center for Infectious Diseases, and assistant professor of the Faculty of Medicine, Laval University, Quebec, Canada in 1999 and is full professor since 2010. She is also director of the Bioimaging platform since 2003.

Junko Nio-Kobayashi
Laboratory of Histology and Cytology, Faculty of Medicine and Graduate School of Medicine, Hokkaido University, Sapporo, Japan. Ph.D, Veterinary Medicine
Junko Nio-Kobayashi graduated from Faculty of Veterinary Medicine, Hokkaido University. She earned her Ph.D. degree from Hokkaido University, Graduate School of Veterinary Medicine, and was appointed Assistant Professor at the Laboratory of Histology and Cytology, Faculty of Medicine and Graduate School of Medicine, Hokkaido University in 2006. She has been engaged in the analysis of galectin-expressing cells in various organs under the guidance of Prof. Toshihiko Iwanaga, who specializes in histology. For two years from 2010, she conducted research on galectins in human corpus luteum and Fallopian tubes in Prof. W. Colin Duncan’s laboratory in the University of Edinburgh, UK, as a JSPS Postdoctoral Fellow for Research Abroad. After returning to Hokkaido University, she became a lecturer in 2018.

Jun Hirabayashi
Tokai National Higher Education and Research System, Nagoya University, Japan. Ph.D, Science.
After graduated from Tohoku University (Master of Science), he started his professional carrier at Teikyo University under Prof. Kenichi Kasai for the investigation of animal lectins. On the occasion of GlycoXV (Tokyo, 1999), he proposed the concept glycome; for this realization, he moved to National Institute of Advanced Industrial Science and Technology (AIST, Tsukuba) in 2002, and was involved in a series of national projects for glycan engineering, while he was a deputy director in Research Center for Medical Glycoscience (2006~), prime senior researcher of Research Center for Stem Cell Engineering (2012~). Now, he is a designated professor in Institute for Glyco-core Research (iGCORE), Tokai National Higher Education and Research System, Nagoya University, while being a vice president of the Japanese Society of Carbohydrate Research (JSCR) and Japanese Consortium for Glycoscience and Glycotechnology. (JCGG). He is also a visiting professor of Kagawa University (2003~) and Yokohama City University (2019~).
This chapter is a “spin-off” version of “Milk oligosaccharides and Galectins” by Urashima and Hirabayashi, published in the main part of the Galectin series (Vol. 24 [2], A3). Here, we chose a few topics that could not be fully dealt with in the main chapter, thereby aiming to advance discussions about important, but unsolved questions of milk oligosaccharides, in a free and active forum with the extended members. We hope you will enjoy this new forum style.
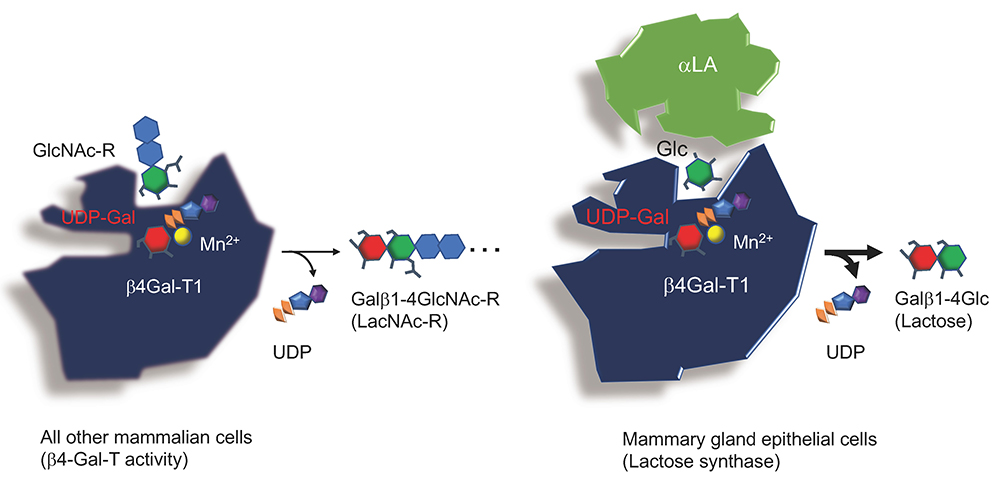
As described in the main chapter, lactose in mammalian milk is biosynthesized as a result of a change in the acceptor substrate specificity of β1,4-galactosyltransferase I (β4Gal-T1), i.e., from N-acetylglucosamine (GlcNAc) to glucose (Glc), triggered by α-lactalbumin (αLA), which exclusively binds to β4Gal-T1 (Fig. 1).
This indicates that the substrate specificity of β4Gal-T1 is stereochemically regulated, and that the affinity of β4Gal-T1 for Glc is greatly increased in comparison with its affinity for GlcNAc, once αLA binds to this enzyme. Thus, it is worth investigating how this “wobbling” of β4Gal-T1 substrate specificity is achieved, in association with acquisition of a new substantial energy source critical for the growth and development of newborn children.
In the following sections, we briefly describe first the scientific background to this topic of wobbling before discussing it.
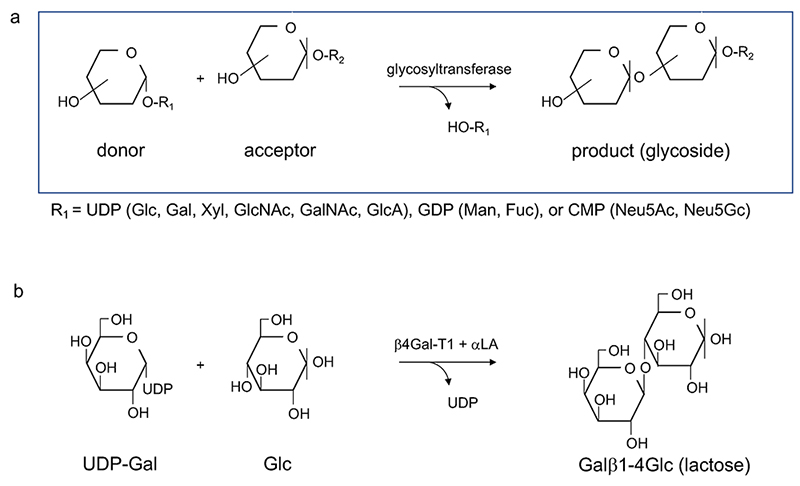
Glycosyltransferase is a generic term given to a group of enzymes, which transfer a saccharide residue. In general, a monosaccharide (donor) is transferred in the form of sugar nucleotide to substances (acceptors), such as oligosaccharides and glycosides (Fig. 2). It is widely known that glycosyltransferases have extremely high substrate specificity, while a wide variety of oligosaccharide structures are found in nature. Thus, this structural diversity was once attributed to molecular recombination of glycosyltransferase, similar to the recombination of immunoglobulins (the so-called Roseman hypothesis) 1,2 (Note 1).
At the end of the 20th century, however, investigators succeeded in molecular cloning of a series of glycosyltransferases, though it had previously been extremely hard to do. As a result, X-ray crystallography analyses revealed detailed molecular mechanisms of glycosyltransferase actions and it became possible to classify large families of glycosyltransferases on the basis of their molecular structures.
Further, results from these analyses suggest the following bases for glycosyltransferase categorization (in order of importance): 1) the types of glycoside linkages they form (e.g., α1-6, β1-3); 2) their specificity for donor substrates; and 3) their specificity for acceptor substrates. In other words, acceptor specificity is a less rigorous basis for categorization than donor specificity.
The above-described rules are largely empirical, and they are consistent with structural data observed to date. Of note, the last point that acceptor specificity is rather loose compared to donor specificity is similar to the situation with a certain class of glycosidases, which possess trans glycosylation activity. Probably, these two groups of enzymes operate on the same important principle.
Note 1: Roseman’s group first identified two oligosaccharide nucleotides as Siaα2-3(6)Galβ1-4GlcNAcα1-UDP and Siaα2-3(6)Galβ1-6GlcNAcα1-UDP in caprine colostrum in 19613. Then, Kobata found that human milk contained Fucα1-2Galβ1-4GlcNAcα1-UDP in 19664. Although he looked for oligosaccharide transfer from this oligosaccharide nucleotide in human milk, it was not discovered until now. Around 50 years later, one of the authors of this chapter, Urashima, identified two oligosaccharide nucleotides, Neu5Gcα2-3Galβ1-4GlcNAcα1-UDP and Neu5Gcα2-6Galβ1-4GlcNAcα1-UDP, in ovine colostrum5. Although sometimes reported, oligosaccharide nucleotides have not been consistently found in milk and colostrum, implying that their presence may vary depending on individual differences or experimental conditions. Even though Urashima’s group failed to isolate the oligosaccharide nucleotides from bovine, ovine, and caprine colostrum again, he successfully isolated UDP-Gal and UDP-Glc from bovine colostrum, and UDP-Gal, UDP-Glc, UDP-GalNAc, and UDP-GlcNAc from caprine and ovine colostrum6. Their efforts have not been well rewarded and the physiological reason for the presence of these oligosaccharide nucleotides remains mysterious.
In addition to causing the well-known molecular “wobbling” of the acceptor specificity in β4Gal-T1, the interaction with αLA appears also to change donor specificity.
Cummings et al. found that GalNAc had been transferred to the acceptor when the β4Gal-T1 from cow’s milk had been incubated with UDP-GalNAc as a donor and GlcNAc as an acceptor in the presence of αLA7. They hypothesized that this reaction was related to the biosynthesis of GalNAcβ1-4GlcNAc (LacdiNAc) at the non-reducing ends of the sugar moieties of some glycoproteins in cow’s milk. Indeed, this structural unit was found in the carbohydrate structures in butyrophilin, which exists in the milk fat globule membrane8,9.
LacdiNAc has been found from a comprehensive glycomics study of bovine colostrum glycoproteins, but not from a glycomics study of human milk10. A trace amount of free LacdiNAc and its analogue GalNAcβ1-4Glc is also found in bovine colostrum11,12. Although it is very interesting to determine if its biosynthesis is related to the modification of the donor specificity of the β4Gal-T1 from UDP-Gal to UDP-GalNAc as a result of interaction with αLA, this effect may be limited at least in humans during the biosynthesis of carbohydrate moieties, since the presence of LacdiNAc in the glycoconjugates is only confirmed in bovine but not human milk/colostrum.
According to the group of Qasba, who has studied β4Gal-T1 as his life’s work, it is likely that β4Gal-T1 has the ability to transfer Glc to GlcNAc at 0.3–0.4% of the strength of Gal, and this ability is enhanced around 30-fold by interaction with αLA13. However, because Glcβ1-4GlcNAc has not been found to occur naturally in carbohydrate units of the glycoconjugate, it is speculated that such modification of the donor from UDP-Gal to UDP-Glc does not occur in vivo. However, the finding that molecular “wobbling” of β4Gal-T1 donors as well as acceptors occurs through its interaction with αLA indeed contributes to a paradigm shift from the rule that the activity of one enzyme is attributable to one gene (Note 2).
Note 2: The theory proposed by Beadle and Tatum in 194114 insists that one gene governs production of one enzyme (protein). The theory was first proposed as a hypothesis based on evidence from a series of experiments using auxotrophic mutants of red bread mold. Though regarded as a foundational dogma of contemporary genetics, the theory has continued to evolve with progress in molecular biology.
As described, the substrate specificity of β4Gal-T1 is substantially changed by association with αLA. For this, detailed studies have been made to elucidate how the binding pocket changes structurally around its acceptor and donor substrates.
Ramakrishnan and Qasba performed X-ray crystallography as well as docking simulation studies, and they observed conformation change in β4Gal-T1 not only with UDP-Gal but also UDP-GalNAc and UDP-Glc15, (Note 3). Thus, as regards to β4Gal-T1, the donor specificity does not seem to be rigorous, because the enzyme showed “wobbling” of not only its acceptor substrate but also its donor substrate.
Here, one question arises: would such a substrate change be observed only in β4Gal-T1? Or would it be a special case since the enzyme is involved in “lactose synthesis”, which mammals successfully acquire? Or are there substances other than αLA that induce change in either a donor or acceptor substrate?
For example, how about invertebrate β4GalNAc-T and ppGalNAc-T, the latter being involved in the initial step of mucin-type glycosylation and are supposed to resemble β4Gal-T structurally? Alternatively, are there relatively small proteins or non-protein molecules that through association with a glycosyltransferase, change its structure and thereby its substrate specificity?
By any chance, haven’t we had an implicit or subconscious prejudice regarding the substrate specificity of glycosyltransferases? Herein, we propose the following hypothesis, even though we know it's a little overwhelming.
If the above hypothesis is true, some glycosyltransferases other than β4Gal-T1 would be expected to have a molecular switch to a different substrate specificity. On the other hand, if such an example is never discovered (it is actually difficult to prove a ‘negative’ event), then the acquisition of the capacity to synthesize lactose in mammary gland epithelia is indeed an exceptional case that has led to the great success of mammals.
Note 3: The difference between the donor substrates UDP-Gal and UDP-GalNAc is the 2-N-acetyl group, while that between UDP-Gal and UDP-Glc is the configuration of the C4-OH group, which is axial in Gal and equatorial in Glc. According to the crystallography analysis, the C4-OH group is recognized by the same amino acid residue (Glu317)15. This is quite notable considering that generally galactose-binding lectins (which include galectins, the theme of this series) never mistake Glc for Gal owing to the establishment of an extensive hydrogen bond network via multiple amino acid residues typically through their hydrophilic side chains. On the other hand, β4Gal-T1 recognizes the donor substrate UDP-Gal through recognition of only a single C4-OH (axial) group. Apparently, this results in less rigorous recognition of the donor substrate.
As described above, glycosyltransferases have the following (from most to least rigorous) requirements: 1) glycoside linkages of different types, 2) specificity for donor substrates, and 3) specificity for acceptor substrates.
According to requirement 3), β4Gal-T1 possibly transfers Gal to various acceptor substrates other than Glc once associated with αLA. Moreover, as hypothesized, such changes in substrate specificity may occur through interaction with an appropriate component other than αLA. The first possibility has been reported. Namely, galactosyl-myo-inositol is found in the milk of some mammals (Fig. 3).
There are 9 inositol isomers, among which myo-inositol is the most abundant in nature. All have the same composition formula as Glc, i.e., C6H12O6. Though distinct from Glc and other monosaccharides, inositol has neither aldehyde nor keto groups, thereby does not fulfill the definition of a carbohydrate. In other words, inositol (also known as 1,2,3,4,5,6-cyclohexanehexaol) is similar to but distinct from saccharides (Note 4).
Note 4: Intriguingly, among inositol isomers, all of which have chiral carbons, only chiro-inositol has enantiomeric (i.e., D and L) forms. Myo-Inositol is biosynthesized from Glc via C5 oxidation (keto formation), keto-enol tautomerism, intramolecular aldol condensation, through which the pyranose ring is converted to the cyclohexane ring16. It is totally unknown how and why this molecule can be an acceptor substrate for β4Gal-T1 instead of Glc and what its benefits (biological functions) are.
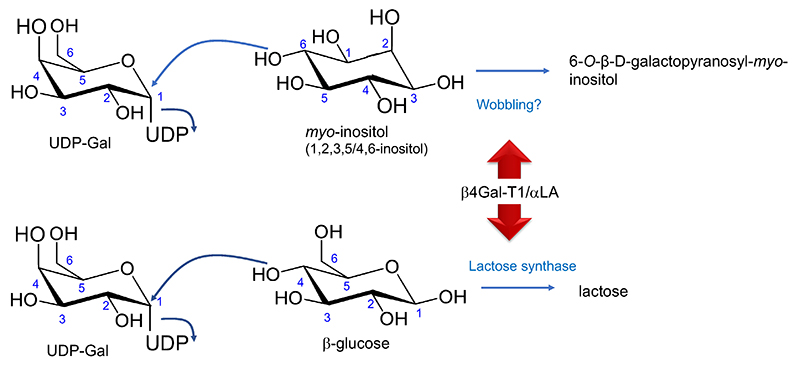
It has been published that rat milk contains a high concentration (0.25 µmol/mL [8.55 mg/100 mL] at 18 days postpartum) of galactosyl-myo-inositol (6-O-β-D-galactopyranosyl-myo-inositol)17. This myo-inositol glycoside had been found first in the extract of lactating rat mammary glands18, and then in the milk but not other tissues17. We hypothesize that this is synthesized due to change in wobbling of the acceptor affinity caused by the interaction of β4Gal-T1 with αLA (Fig. 2). Galactosyl-myo-inositol was indeed synthesized by transfer of Gal from UDP-Gal to myo-inositol when in the presence of the Golgi apparatus membrane fraction used as an enzyme source and αLA19.
It has been reported that the milks of rodents, including house rabbits, contain a high concentration (~30 mg/100 mL) of myo-inositol (not galactosyl-myo-inositol), probably derived from the diet20. When animals were fed diets without myo-inositol, this concentration in milk decreased to 10 mg/100 mL20. It is thought that dietary myo-inositol must have been absorbed in the small intestine, entered the circulation, and traveled to the mammary glands. Some of this component was carried by the blood and transported across the basal membranes of mammary epithelial cells where it was incorporated.
Whether myo-inositol can enter the binding pocket of β4Gal-T1 in the presence of αLA would be clarified by conducting a docking simulation of the complex αLA-β4Gal-T1 with myo-inositol. Galactosyl-myo-inositol was detected also in swine colostrum by Prof. Tadao Saito’s group at Tohoku University, Graduate School of Agriculture (T. Saito, unpublished result).
When Urashima studied the milk oligosaccharides of many mammalian species, he found on a few occasions glycoside-like components that were neither Glc nor other monosaccharides at the reducing-ends. For example, he found a such type compound containing A antigen [GalNAcα1-3(Fucα1-2)Gal] in the milk of sloth bears (Urashima, unpublished result). It is speculated that the precursor of this compound would be influenced by the “wobbling” of β4Gal-T1 causing it to transfer Gal to a non-sugar component originating in the diet of the sloth bear.
A clear difference between lactose and galactosyl-myo-inositol is that the latter lacks both reducing activity and reactivity with amines. Inositol is also present in glycosyl-phosphatidyl-inositol (GPI)-anchored proteins, but the reason inositol is used remains unknown. How mysterious a material this inositol is!
The diversity that life systems produce seems almost infinite. Moreover, the systems are not restricted by dogmas learned in the past, such as the “lock and key” and “one gene and one enzyme” theories. Discussion we have made in this spin-off version include a proposed hypothesis that there are still unidentified molecular switches in many other proteins, which are possibly triggered by molecules like αLA. To test this possibility, we must carefully check whether actual features of extensive biomaterials in our hands, e.g., their in vivo compositions and proportions, could totally represent those predicted from genomic information. Namely, if we find some unpredicted materials or apparently biased product formation, it may be a clue to the occurrence of unidentified gene(s) or the presently discussed “molecular switch”.
As a result of interaction with αLA, it is also demonstrated by molecular simulation that β4Gal-T1 wobbling is not directly limited to lactose but also various other materials. Among the compounds whose biosynthesis could be simulated, only lactose, which is universally present in mammals, has an important function as a nutritional source for suckling neonates; this is no doubt a selective advantage during the mammalian evolution. Also, in other cases, it should be a novel aspect in the evolutional biology to explorer the nature of selective advantage for the survival brought by a new component, which could have been synthesized by the effect of molecular “wobbling”, i.e., modification of the substrate caused by steric effects between some enzymes and other components.
Acknowledgments The authors thank the following scientists who gave useful information and hints to help the present discussion: Taroh Kinoshita (Osaka University), Kensei Kobayashi (Yokohama National University), Hisashi Narimatsu, Takashi Sato, Tomomi Kubota (National Institute of Advanced Industrial Science and Technology), and Olav Oftedal (Smithsonian Environmental Institute).