
Tadasu Urashima
Obihiro University of Agriculture & Veterinary Medicine, Obihiro, Japan. Ph.D., Agriculture.
After graduation from Tohoku University (Doctor of Agriculture) in 1986, he started his professional career by studying milk oligosaccharides at Obihiro University. In 1991, he studied glycosyltransferase activity in lactating mammary glands of the tammar wallaby (marsupial) under Dr. Michael Messer in the Department of Biochemistry, the University of Sydney. Then he completed a comprehensive study of milk oligosaccharides of monotremes, marsupials, and several species of eutherians with Dr. Messer. He is interested in how the present milk components have been acquired during the evolution of mammals, especially how the acquisition of α-lactalbumin, a milk protein, has resulted in the appearance of milk oligosaccharides and lactose, and caused their biological significance to change during evolution. Since 2003, he has been a full professor at Obihiro University. At present he is president of the Japanese Society of Dairy Science and a board member of the Japanese Society of Carbohydrate Research (JSCR) and Japanese Consortium for Glycoscience and Glycotechnology (JCGG).

Sachiko Sato
Research Centre for Infectious Diseases, Faculty of Medicine, Laval University, Quebec City, Canada. Ph.D, Pharmaceutical science
Sachiko Sato graduated from Faculty of Pharmaceutical Science, Chiba University. She joined as postgraduate student in the laboratory of Dr. Akira Kobata, the Institute of Medical Science, the University of Tokyo, Japan in 1987. She also worked in the laboratory of Dr. R. Colin Hughes, MRC: National Institute for Medical Research in London, UK, where she first encountered a cytosolic mammalian lectin, now called galectin-3. She obtained her Ph. D. from the University of Tokyo in 1994. As postdoctoral fellow in the laboratory of Dr. Ron Kopito, Stanford University, she was involved in the work on cystic fibrosis. She became principal investigator of the laboratory of glycobiology in Research Center for Infectious Diseases, and assistant professor of the Faculty of Medicine, Laval University, Quebec, Canada in 1999 and is full professor since 2010. She is also director of the Bioimaging platform since 2003.

Junko Nio-Kobayashi
Laboratory of Histology and Cytology, Faculty of Medicine and Graduate School of Medicine, Hokkaido University, Sapporo, Japan. Ph.D, Veterinary Medicine
Junko Nio-Kobayashi graduated from Faculty of Veterinary Medicine, Hokkaido University. She earned her Ph.D. degree from Hokkaido University, Graduate School of Veterinary Medicine, and was appointed Assistant Professor at the Laboratory of Histology and Cytology, Faculty of Medicine and Graduate School of Medicine, Hokkaido University in 2006. She has been engaged in the analysis of galectin-expressing cells in various organs under the guidance of Prof. Toshihiko Iwanaga, who specializes in histology. For two years from 2010, she conducted research on galectins in human corpus luteum and Fallopian tubes in Prof. W. Colin Duncan’s laboratory in the University of Edinburgh, UK, as a JSPS Postdoctoral Fellow for Research Abroad. After returning to Hokkaido University, she became a lecturer in 2018.

Jun Hirabayashi
Tokai National Higher Education and Research System, Nagoya University, Japan. Ph.D, Science.
After graduated from Tohoku University (Master of Science), he started his professional carrier at Teikyo University under Prof. Kenichi Kasai for the investigation of animal lectins. On the occasion of GlycoXV (Tokyo, 1999), he proposed the concept glycome; for this realization, he moved to National Institute of Advanced Industrial Science and Technology (AIST, Tsukuba) in 2002, and was involved in a series of national projects for glycan engineering, while he was a deputy director in Research Center for Medical Glycoscience (2006~), prime senior researcher of Research Center for Stem Cell Engineering (2012~). Now, he is a designated professor in Institute for Glyco-core Research (iGCORE), Tokai National Higher Education and Research System, Nagoya University, while being a vice president of the Japanese Society of Carbohydrate Research (JSCR) and Japanese Consortium for Glycoscience and Glycotechnology. (JCGG). He is also a visiting professor of Kagawa University (2003~) and Yokohama City University (2019~).
In the main chapter of this series, “Milk oligosaccharides and galectins” (Glycoforum. 2021 Vol. 24 (2), A3), we commented that rat milk includes largely 3’-sialyllactose (3’-SL) and 6’-sialyllactose (6’-SL)1,2, while that of another rodent species, the lowland paca (Cuniculus paca), possesses more complex structures of milk oligosaccharides. This observation inspires us to postulate that the milk oligosaccharides of experimental animals are much simpler than those of wild animals. However, it was recently reported that rat and mouse milks contain a small amount of more complex oligosaccharides, such as sulfo 3’-sialyllactose3. Here, we draw attention to another unsolved question as a theme for this second spin-off version: how is the structural diversity of milk oligosaccharides defined?
The structures of milk oligosaccharides of pacas are shown in Fig. 1. While the number of determined structures, 7, is not large, their structures are very unique, i.e., GalNAcβ1-4(Neu5Acα2-3)Galβ1-4Glc (GM2 tetrasaccharide), Neu5Acα2-3Galβ1-3GalNAcβ1-4Galβ1-4Glc (GM1b pentasaccharide), Neu5Acα2-3Galβ1-3(Neu5Acα2-6)GlcNAcβ1-3Galβ1-4Glc (disialyllacto-N-tetraose; DSLNT), Neu5Acα2-3Galβ1-3GalNAcβ1-4(Neu5Acα2-3)Galβ1-4Glc (GD1a hexasaccharide), and Galβ1-3Galβ1-4Glc (3’-GL) (Note 1).
Note 1: Urashima, one of the authors, had encountered an unexpected error in the study of paca milk oligosaccharides. After fractionation of milk oligosaccharides by ion exchange chromatography, the solution had been stored in alkaline buffer in a refrigerator, resulting in the isomerization of the reducing ends of the oligosaccharides. As this limited the quantification of oligosaccharides, the authors suspended the data preparation needed for submission as a manuscript. This reaction appears to be a result of Lobry de Bruyn rearrangement, possibly occurring under basic conditions. Namely, the reducing terminal of Glc residues of the oligosaccharides presumably are isomerized via a 1,2-enediol intermediate to fructose and mannose residues. Lobry de Bruyn rearrangement, as described in textbooks, is a rather specific event that applies to monosaccharides, while in principle, even oligosaccharides should undergo the reaction and there are no reports on the subject of Lobry de Bruyn rearrangement in oligosaccharides. Thus, in this sense, we think that the above observation deserves specific attention.

DSLNT has been also found in human milk and is recognized as one of the predominant acidic oligosaccharides in human milk4 (Note 2).
Note 2: It is known that risk of necrotizing enterocolitis (NEC), which sometimes causes serious damage in preterm infants, is lower in breast-fed than in formula-fed infants. It is suggested that DSLNT has the specific effect of preventing or treating NEC according to in vivo experiments with a rat model of NEC5.
Urashima reported that in the milks of most mammals, with the notable exception of humans, type II oligosaccharides containing Galβ1-4GlcNAc predominate over type I oligosaccharides containing Galβ1-3Glc6. As shown in DSLNT structure, which contains a type I unit, type I oligosaccharides predominate in the milk of paca, similar to the milk of humans.
The chemical structures of GM2 tetrasaccharide, GM1b pentasaccharide, and GD1a hexasaccharide are similar to those of the ganglio series glycolipids. GM2 tetrasaccharide has been found in the milks of bottlenose dolphins7, giraffes8, and rhesus macaques9, while GM1b pentasaccharide has been detected in goat milk10. Asialo GM2 trisaccharide and asialo GM1tetrasaccharides, in addition, have been found in the milks/colostra of cows10, water buffalos10, goats10, and lions10. Notably, the existence of ganglio type milk oligosaccharides is relatively rare, compared with those of neolacto and lacto type saccharides. The phylogenetic tree of the rodents is shown in Fig. 2. Even though rodents are one of the most prevalent mammalian groups of species, the milk oligosaccharides of most rodents have not been studied so far.
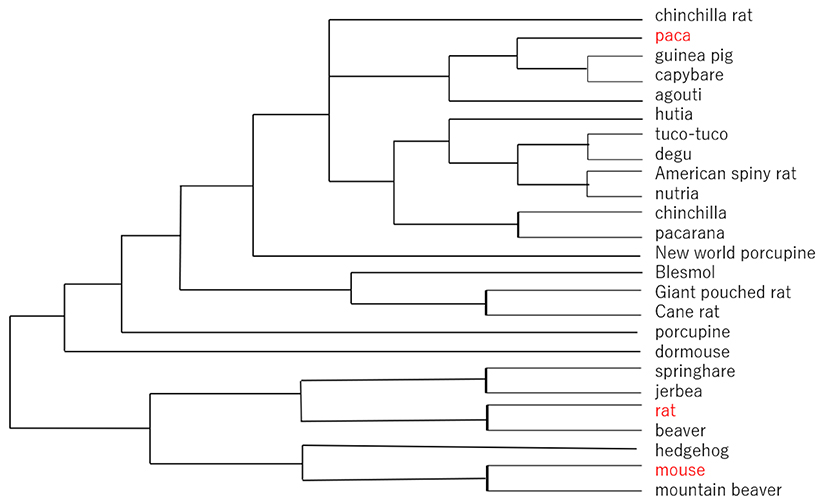
As described above, experimental animals such as rats apparently produce structurally simplified milk oligosaccharides. If we get into a deeper investigation on this point, one of the best approaches would be to use wild rats to compare their milk oligosaccharide structures with those of rats reproduced under well-controlled conditions. Obviously, this experiment would be not so easy to perform.
When comparing the laboratory rat with the wild rodent paca, most of the milk oligosaccharides other than 3’-SL and 6’-SL seem to be largely lost in laboratory rats. Would this mean that complex oligosaccharides become non-essential, because the animals have been kept in well-controlled food and sanitary environments over the years of being bred by humans?
In rat milk, the concentration of sialyllactose is estimated to be 16–14 g/L (26–22 mM) on postpartum days 4 to 8, and the ratio of sialyllactoses to lactose is as high as 0.61–0.5111. It is likely that the biosynthesis of non-sialyl milk oligosaccharides might have been suppressed by increased activity of sialyltransferase in the lactating mammary glands.
The similar phenomenon has been observed in milk oligosaccharides of house dogs, which are not experimental animals but have been bred by humans for centuries. Namely only 2’-FL, 3’-SL, and 6’-SL have been found in the milk of house dogs12 (Note 3). In contrast, the milks of raccoons, bears, minks, and skunks, which are closely related species to dogs among carnivora, contain a variety of oligosaccharides, many of which have highly complex structures13.
Note 3: A tetrasaccharide, GalNAcα1-3(Fucα1-2)Galβ1-4Glc, has been detected in the milk of some breeds of house dog12.
Even though the data are still limited, one might easily hypothesize that decrease in the variety of milk oligosaccharides through structural simplification in the milks of animals being bred by humans for many decades, including experimental and domestic animals, is linked with elevated sialylation. Because sialylation often stops elongation and branching of the sugar chains from lactose, this must be an effective strategy to “simplify” the oligosaccharide structures in milk. H type fucosylation can also suppress the elongation and branching of the carbohydrate chains from lactose.
Improved sanitary environments reduce the frequency of exposure to microbes. It is possible that adoptive evolution under artificial conditions has an impact on the activities or other properties of transferases involved in sialylation and fucosylation, leading to the suppression of elongation and/or branching events during oligosaccharide biosynthesis (Note 4).
Note 4: Such examples of simplified synthesis of oligosaccharides can be found in other biological systems. In natural silk worms, which are cultivated for many generations under artificial environments, the middle silk gland, responsible for exclusive production of silk proteins (e.g., fibroin), completely lacks the insect-specific core α1-3fucose (Fucα1-3GlcNAc) structure14. Considering that all other tissues investigated express core α1-3fucose, it is assumed that evolutionary pressure to not work or become less active has been exerted on enzymes not involved in silk fiber biosynthesis, such as the enzyme responsible for expression of core α1-3fucose. To address this issue, one could investigate glycan structures of the middle silk gland of wild silk worms.
In the main chapter, “Milk oligosaccharides and galectins”, we proposed and showed the biosynthetic pathways of human milk oligosaccharides (HMOs)1. However, one should consider that all of the glycosyltransferases involved in the synthesis are not directly cloned from mammary gland cells. Notably, a gene for β3Gal-T, responsible for the biosynthesis of 3’-GL (Galβ1-3Galβ1-4Glc) remains to be identified (unidentified 1 in Fig. 3).
In other words, a biosynthesis map for the oligosaccharides in human milk as well as those in other animal milks has not been completely elucidated, because not all the key enzymes (genes) have been identified as yet. Indeed, glycosyltransferase activity has been reported in one study using an enzyme source with homogenate derived from mammary glands of the tammar wallaby, a marsupial to measure β3Gal-T, β4Gal-T, and β6GlcNAc-T (IGnT) activity by a conventional biochemical method15,16.
The structural diversity of milk oligosaccharides is substantially due to the presence of IGnT. In this context, one of the biosynthetic pathways has not been described in Fig.2 in the main chapter, because it corresponds to a minor pathway1. For example, a trace level of Neu5Acα2-3Galβ1-3[Galβ1-4(Fucα1-3)GlcNAcβ1-6]Galβ1-4Glc, which is derived from lacto-N-novopentaose 1 [Galβ1-3(Galβ1-4GlcNAcβ1-6)Galβ1-4Glc, novoLNP1] as a precursor, has been identified as an HMO17. It is thought that novoLNP1 is synthesized by the action of an unidentified β6GlcNAc-T, which has a different substrate specificity from the known IGnT (Note 5).
Note 5: Urashima found that this type of β6GlcNAc-T is active in tammar wallaby lactating mammary glands. He studied the activity of this β6GlcNAc-T with the acceptor 3’-GL or Galβ1-3Galβ1-3Galβ1-4Glc (digalactosyllactose; DGL) and the donor UDP-GlcNAc, using homogenates of lactating tammar wallaby mammary glands as an enzyme source, at the University of Sydney under Michael Messer in 199116. This transferase can use not only 3’-GL but also DGL, for which GlcNAc is transferred to the penultimate Gal from non-reducing ends via β1-6 linkage to form Galβ1-3(GlcNAcβ1-6)Galβ1-4Glc and Galβ1-3(GlcNAcβ1-6)Galβ1-3Galβ1-4Glc, respectively. Moreover, Galβ1-3(Galβ1-4GlcNAcβ1-6)Galβ1-3Galβ1-4Glc synthesized from the pentasaccharide by the activity of β4Gal-T, has been, indeed, identified in the milks of the marsupials, brushtail possum and eastern quoll18,19. However, the molecular biology of cloned mammary epithelial cell genes that encode glycosyltransferases related to the biosynthesis of oligosaccharides remains to be studied.
Namely, GlcNAcβ1-3Galβ1-4Glc (lacto-N-triose-Ⅱ) is the substrate for known IGnT, whereas this β6GlcNAc-T transfers GlcNAc from UDP-GlcNAc to the penultimate Gal of 3’-GL (Galβ1-3Galβ1-4Glc) via β1-6 linkage (see unidentified 2 in Fig. 3, which is related to the biosynthesis of lacto-N-novotetraose, novoLNT).
To validate this, first of all, we need to identify the IGnT gene, and then check whether it can discriminate between monosaccharides (GlcNAc and Gal) linked at C3-position of Gal in lactose. If the enzyme shows reactivity to one of them, it means there is an unidentified enzyme with specificity for the other monosaccharide. If the enzyme shows reactivity to both, it means the transferase is bifunctional.
NovoLNP1 and its derivatives are major branch type oligosaccharides, in contrast to milk oligosaccharides in humans, and they are also found in the milks/colostra of many eutherians. In fact, these have been detected in the milks/colostra of cows20,21, horses21,22, goats21, sheep21, pigs21, camels21,23 and tufted capuchin monkeys (one of the New world monkeys)24, showing that the structures in milks/colostra are common to marsupials and some species of eutherians.
Among eutherians, novoLNP1 predominates over lacto-N-neotetraose (LNnT) and lacto-N-neohexaose (LNnH) in horse colostrum, whereas its concentration is similar to that of LNnT in the colostra of cows, goats, sheep, pigs, and camels21. LNnT and LNnH, which are major oligosaccharides in many eutherian milks/colostra, have never been found in the milks of marsupials. This means that transferases in marsupial mammary glands do not have iGnT activity, or this enzyme is almost inactive.
In other words, which core structure novoLNP1 or LNnT in milk oligosaccharides is largely dependent on which enzyme is more dominant; namely, β3GalT or iGnT, which are responsible for the biosynthesis of “3’-GL” and “lacto-N-triose-Ⅱ”, respectively (Fig. 3).
The observation that novoLNP1 is frequently found even in Placentalia implies that β3Gal-T appeared earlier in the course of evolution (Note 6). The identification of β3Gal-T, an enzyme responsible for 3’-GL biosynthesis, remains an unsolved issue.
Note 6: Whether LNnT or LNnH predominates over novoLNP1 or vice versa varies depending on mammalian eutherian species. Roughly estimated, levels of LNnT/LNnH and novoLNP1 are likely to be equal in artiodactyla and LNnT/LNnH is likely to predominate over novoLNP1 in primates and carnivora, whereas the ratio of novoLNP1 is relatively high in perissodactyla.
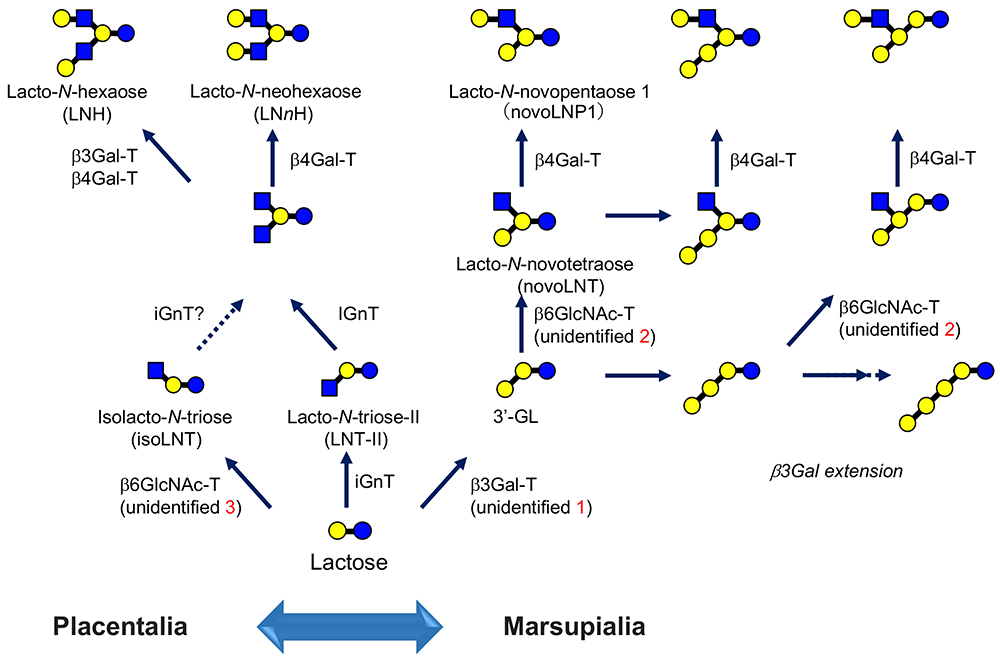
Possibly, there is still another β6GlcNAc-T with specificity that is different from those described so far (“unidentified 3” in Fig. 3). Galβ1-4GlcNAcβ1-6Galβ1-4Glc (isolacto-N-neotetraose) was discovered for the first time by one of the authors (Urashima)25, and has been shown to be more widely present in cows21, goats21, sheep21, camels21, pigs21, buffalos10, and lions10.
This structure is biosynthesized from the precursor GlcNAcβ1-6Galβ1-4Glc (isolacto-N-triose). Here, please note that the known IGnT recognizes lacto-N-triose-Ⅱ as a specific acceptor and introduces a “β1-6 branch”; thus, the enzyme is not likely to recognize lactose (in this case, not to make a new branch but just to extend a β1-6 linkage). If this is the case, isolacto-N-triose should be synthesized by a third β6GlcNAc-T, i.e., isolacto-N-triose synthase.
The type II lactosamine units are synthesized from core structures, whose biosynthesis is catalyzed by the three β6GlcNAc-Ts described above, i.e., IGnT, lacto-N-novotetraose synthetic transferase, and isolacto-N-triose synthetic transferase, and the production of type II lactosamine is catalyzed by β4Gal-T. Also, further elongation and branching of sugar chains to form more complicated core structures are catalyzed by iGnT and β6GlcNAc-T.
The ratio of type I to type II oligosaccharides should be determined by the level of β3Gal-T and β4Gal-T activity, and the synthesis of the sugar units of H antigen (Fucα1-2Gal)Gal, Lewis a [Galβ1-3(Fucα1-4)GlcNAc], Lewis x [Galβ1-4(Fucα1-3)GlcNAc], A antigen [GalNAcα1-3(Fucα1-2)Gal], B antigen [Galα1-3(Fucα1-2)Gal], and α-Gal epitope (Galα1-3Gal) should be catalyzed by several fucosyltransferases, α3galactosyltransferase, and α3N-acetylgalactosaminyltransferase, which transfer Fuc, Gal, and GalNAc, respectively, to the non-reducing ends to form milk oligosaccharides containing various sugar epitopes.
Needless to say, it is important to clone cDNAs for mammary gland genes that encode glycosyltransferases specific for the chemical structures of oligosaccharides identified in milk. This would contribute greatly to elucidating a variety of biological phenomena associated with milk oligosaccharides (immunomodulation with various endogenous lectins, prebiotics in bacterial flora in the intestine, innate immune defenses against viral infection, etc.). Once the responsible enzymes (genes) are identified, their substrate specificities as well as specific activities will be clarified, which opens the way to predict the level of oligosaccharide products and to control industrial procedures for the synthesis of oligosaccharide products in bioindustry. Moreover, molecular improvement of these enzymes will be performed with assistance of informatics.
It is concluded that milk oligosaccharides function as both nutritional and informative components. As they would be useful as model components to study the biological reactions between the glycoconjugates and lectins, they must have the strength with some aspects. Many people are interested in them as prebiotics and potential galectin inhibitors, i.e., materials for developing drugs. The trial to prepare, on an industrial level, human type milk oligosaccharides and incorporate them in infant formulas with bovine milk, which only contains trace amounts, is progressing. Although only a few oligosaccharides are available at this stage, we believe that basic scientific information about them is essential to further develop their industrial applications.