
Masao Nakamura
Research Scientist, Sasaki Institute, Sasaki Foundation
Doctorate (Science), Ibaraki University, 2012
Technical Trainees, AIST, from April 2007
Research Scientist, RIKEN Brain Science Institute, from April 2012
Assistant Professor, Tokyo University of Technology, from April 2016
Current position from April 2020
Poster Award at the annual meeting of the Japan Society of Carbohydrate Research, 2012
Highest Award at the 4th meeting of deciphering sugar chain-based signals integrative neuronal functions, Grant-in-Aid of Scientific Research on Innovative Areas, 2013
Poster Award at the GlycoTOKYO 2017, 2017
Lactoferrin (LF) is a glycosaminoglycan-binding protein that functions in innate immunity, and it is expected to be useful as a biopharmaceutical product due to its effects on the body. We aim to develop novel therapeutic agents that can promote effective recovery from spinal cord injuries. Through our recent research, we discovered that LF binds to chondroitin sulfate E (CS-E), inhibiting nerve axon outgrowth and neutralizing its toxicity. We have also developed a highly functionalized neuroprotective molecule consisting of granulocyte colony-stimulating factor (G-CSF) linked to LF. This paper introduces this highly functionalized LF and discusses its glycan-binding and neuroprotective properties.
LF was first identified in milk by Sorensen and isolated as a red protein from the whey of bovine milk1. LF is an iron-binding glycoprotein with a molecular weight of approximately 80,000, and it is found in exocrine secretions such as colostrum, tears, and sweat, as well as in neutrophils. LF has two globular domains, designated N-lobe and C-lobe, each with the ability to bind one trivalent iron molecule (Fig. 1A). The conformation of the N-lobe side of LF can change substantially depending on the degree of iron binding.
In vivo, LF is a protein that bridges mammalian innate and adaptive immune functions, and its protective effects have been the subject of multifaceted research on topics ranging from anticancer, anti-inflammatory, and immunomodulatory activities to antimicrobial activity against numerous microorganisms (Fig. 1B). This breadth of LF activity is due not only to its iron-binding capacity, but also to a mechanism of action involving LF interaction with pathogens and cellular components.
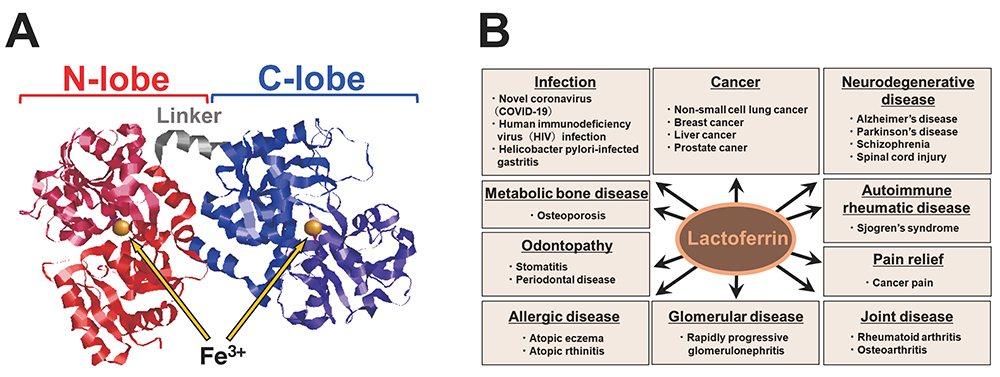
Glycosaminoglycans (GAGs) have been reported to regulate cell motility, cell adhesion, and cell proliferation by interacting with a variety of humoral factors 2. GAGs are linear molecular structures consisting of repeating disaccharide units of uronic acid and amino sugars, and are found on cell surfaces and in extracellular matrices. GAGs are broadly classified into various types, including heparin (HP), heparan sulfate (HS), and chondroitin sulfate (CS), depending on their disaccharide structure, and are further classified into several subtypes according to the position of the sulfate group (Fig. 2A). To date, more than 700 proteins have been identified as interacting with HP, including LF, which was shown to bind HP in 1980 3. It has been reported that LF has three GAG binding sites in its N-terminal region (Fig. 2B). LF has been shown to be effective in 1) protecting dopamine neurons from damage in Parkinson's disease models, 2) inhibiting coronavirus infection associated with severe acute respiratory syndrome, and 3) inhibiting the proliferation of human breast cancer cells. As such, LF is expected to be used for treatment of GAG-related diseases (Table. 1).
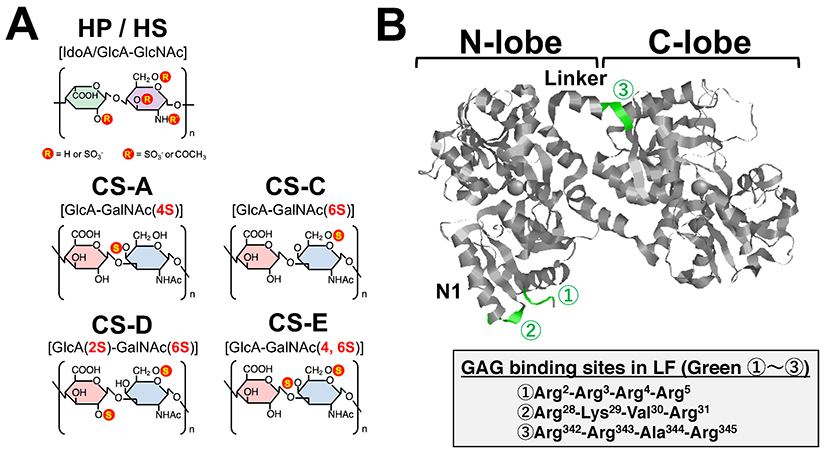
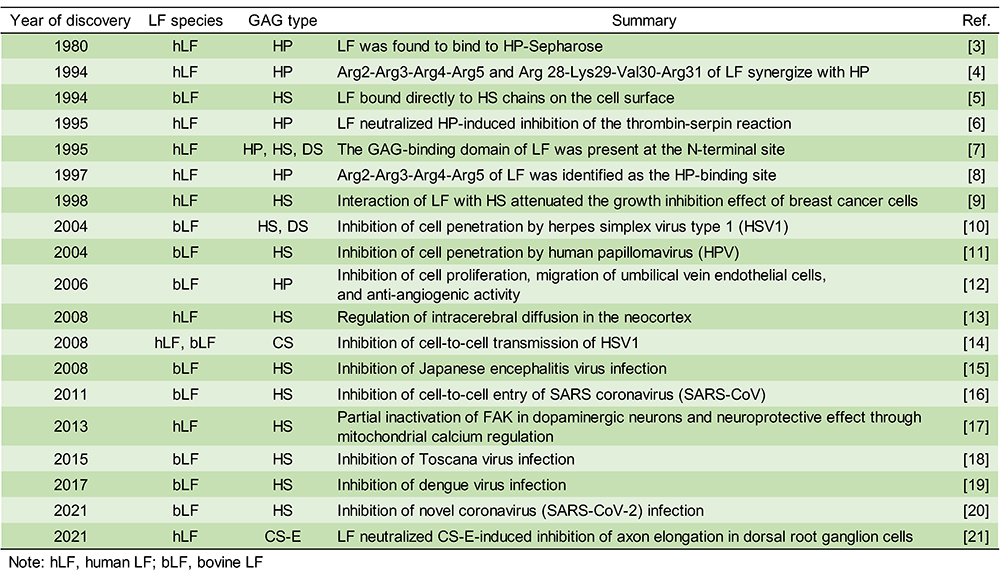
In recent years, it has been reported that the binding between LF and HS plays an important role in infectious diseases, cancer, and neurodegenerative diseases. However, the binding of LF to glycosaminoglycans other than HP and HS is poorly understood. We analyzed in detail the binding between CS and LF, which controls neural function, and found a new glycosaminoglycan unit that binds to LF 21.
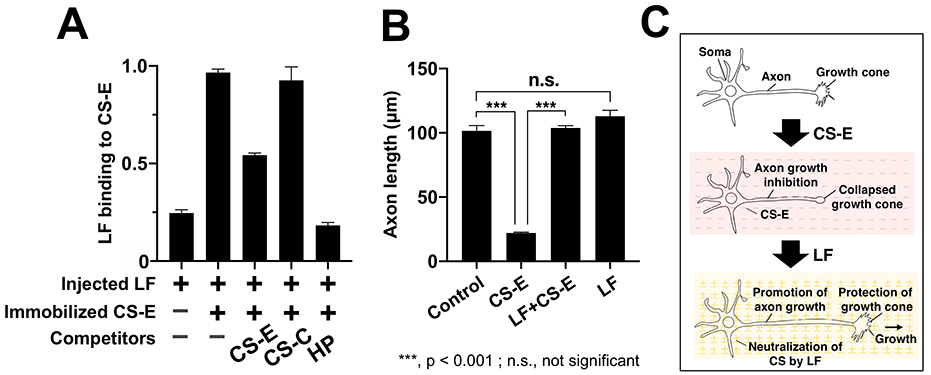
First, we observed the secondary structure of LF in the presence of four CS subtypes (CS-A, CS-C, CS-D, CS-E) and found that the secondary structure of LF changes significantly in the presence of CS-E. Next, to evaluate the binding between LF and CS-E, we prepared CS-E immobilized plates by adding biotinylated CS-E to plates coated with neutral avidin. To these plates, we added LF, incubated the plates for 4 h at 25℃, added a horseradish peroxidase-labeled anti-LF antibody and the chromogenic substrate solution tetramethylbenzidine, and measured absorbance at 450 nm. Our results indicated that LF bound directly to CS-E (Fig. 3A). When HP, CS-C, and CS-E, each in the presence of LF, were allowed to react with the CS-E immobilized plate, competitive inhibition was observed between HP and CS-E, but not with CS-C (Fig. 3A).
We explored how the binding between LF and CS-E could be used in the future. CS-E has been reported to be an axon regeneration inhibitor during spinal cord injury and is anticipated to be a promising drug candidate. The results of the axon growth assay showed that the inhibition of axon growth by CS-E was completely suppressed in the presence of LF, and that axon length was restored to its original state by nullifying the effect of CS-E (Fig. 3B, 3C). As LF alone did not promote axon growth, it was suggested that this effect was merely due to neutralization of CS-E-induced axon growth inhibition (Fig. 3B, 3C).
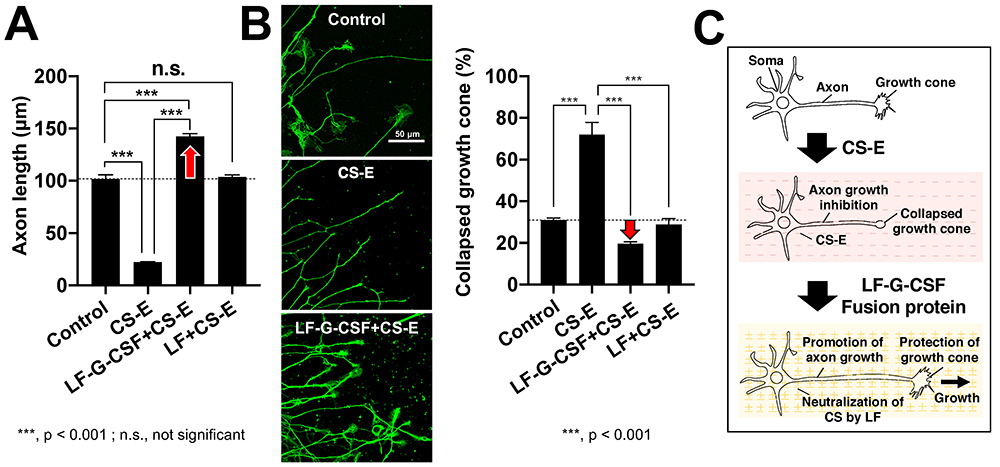
There has been remarkable progress in the development of therapeutic agents for spinal cord injuries. G-CSF has been reported to bring about neuroprotective action through 1) suppression of nerve cell apoptosis 22, 2) suppression of oligodendrocyte cell death and protection of myelin sheath 23, and 3) suppression of inflammatory cytokines 23. We produced a fusion protein of LF and G-CSF (LF-G-CSF), and evaluated its effect. The results of the axon growth assay showed that axon growth inhibition by CS-E was halted by LF and LF-G-CSF, but this effect of LF-G-CSF was greater than that of LF alone (Fig. 4A, 4C). The growth cone at the tip of the axon is a structure found in elongating axons; when the growth cone collapses, the axon growth stops. From the results of the growth cone collapse assay, we determined that LF-G-CSF is more protective than LF against CS-E-induced collapse of the growth cone (Fig. 4A, 4C).
According to the World Health Organization, approximately 250,000 - 500,000 people worldwide are affected by spinal cord injury each year 24, but a cure remains elusive. Neurodegenerative diseases such as spinal cord injury are a concern in Japan because of the increasing number of patients who are aging and at increased risk, and the great need to develop therapeutic agents. We found that LF, a type of HP-binding protein, binds to CS-E and neutralizes axon growth inhibition. By fusing G-CSF and LF, which have neuroprotective effects, we succeeded in developing a highly functionalized LF that suppresses the collapse of the neuronal growth cone caused by CS-E and promotes axon elongation. In the future, we would like to advance scientific knowledge on the functional recovery of nerves using an animal model of spinal cord injury and contribute to the development of therapeutic agents for spinal cord injury targeting CS-E.