
Satoshi Tanimoto
Associate Professor, Department of Materials Science, School of Engineering, University of Shiga Prefecture. Ph.D. (Engineering)
Obtained his Ph.D. in polymer chemistry from Kyoto University Graduate School of Engineering in 1998. From 1998 to October 2002, Assistant at the Graduate School of Materials Science, Japan Advanced Institute of Science and Technology. Engaged in research on the microphase-separated structure of crystalline block copolymers. From November 2002, Lecturer at the University of Shiga Prefecture. A common theme of his research involves environmentally friendly polymer materials. He has held his current position since July 2010. More recently, his research has focused on the micronization and functionalization of chitosan.
Chitin and chitosan, the structural polysaccharides that support the exoskeletons of marine organisms such as shrimp and crabs, are the second most abundant biomass after cellulose, but are yet to be fully utilized as natural resources. Various approaches to create high value-added materials using chitin and chitosan have been evaluated, and potential strategies for giving such materials greater functionality include chemical modification and increasing the complexity of shape or structure. In this paper, I will describe a method for preparing a new functional material by forming chitosan into microparticles and combining them with an inorganic material, calcium carbonate.
Microplastics, the fine polymer particles that are mainly found in the Earth’s hydrosphere, have recently become a major environmental problem. They range in size from about 5 millimeters down to 100 nanometers, and are mostly composed of waste polymers that have been degraded or broken down. As the environmental burden of microplastics continues to increase, their impact on the ecosystem has become a cause for concern, and various measures have been proposed to address the problem. These measures can be divided into two broad categories: (a) eliminating plastic waste, the source of microplastics, and (b) using biodegradable plastics. Various approaches in the former category have already been adopted, mainly by governments. In Shiga Prefecture, the home of our university, for example, trash cleanup activities are held along the shore of Lake Biwa.
Meanwhile, biodegradable plastics have been widely regarded as environmentally-conscious options, even before microplastics became a concern. Polylactic acid, often cited as a representative example of biodegradable plastics, is currently being used in agricultural films and similar applications, but because it is a crystalline polymer, it has poor mechanical properties and has yet to replace general-purpose polymers such as polystyrene. In addition, polylactic acid only decomposes in the environment under relatively limited conditions, and investigations of its use as a biodegradable material have therefore become a lower priority. Recently, polylactic acid has gained recognition as a carbon-neutral material, given that plant-derived raw materials are used for its production, and research is underway on its use as a material. Other biodegradable polymers derived from petroleum raw materials are undergoing evaluation in research projects, but they are not carbon neutral.
When choosing biodegradable materials from a carbon-neutral perspective, we cannot rule out natural polymers. Cellulose is one typical example of a natural polymer that has a long history of use as a material. It is a polysaccharide that plays a structural role in supporting plants, and its shape is firmly maintained by hydrogen bonds derived from the numerous hydroxy groups in the molecular chain. Cellulose has been researched extensively and is expected to gain greater utilization in the future1, but I will omit any further mention here.
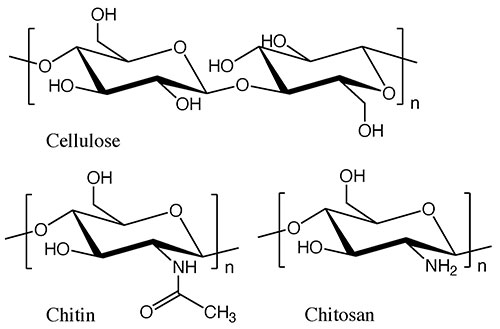
While cellulose is a typical structural polysaccharide, there are other substances that are produced in nature in comparable quantities to cellulose. These are chitin and chitosan, the structural polysaccharides that support the exoskeletons of shrimp and crabs. Chitin has a structure in which the 2-hydroxy group of cellulose is replaced by an acetamide group, and in chitosan, that group is replaced by an amino group (see Fig. 1). In the natural world, chitin and chitosan are found as mixtures in certain proportions, so they are often referred to collectively as chitin-chitosan. They constitute marine biomass that is second only to cellulose in terms of production, but which is still not widely used. One reason for this is that unlike cellulose from wood, chitin-chitosan is contained in the exoskeletons of small marine animals, making it difficult to collect in large quantities.
Our research group has decided to set its focus on chitosan. Chitosan is made by chemically processing chitin-chitosan extracted from shrimp and crabs, and deacetylating the acetamide group to yield only the chitosan component. The molecular structure of chitosan is similar to that of cellulose, but the major difference is that the monomer unit has an amino group. And it is these amino groups that give rise to a variety of useful properties. For example, when chitosan is used as an adsorbent, the amino groups interact with metal ions2. It can also be used as a base material for functional polymers because the high reactivity of the amino group allows for a variety of chemical modifications. Furthermore, due to the antibacterial properties of the amino groups, chitosan is also being considered for medical applications. For example, its use as a wound dressing has been extensively evaluated3.
Chitosan can be used as a bulk material as is, but it is also economically advantageous to fabricate it into a higher value-added material by giving it shape. Therefore, our group has investigated various particle shapes over a long period of time. The most significant difference between chitosan and other polysaccharides is the presence of amino groups, which allows it to be used as a cationic polysaccharide material. A “particle shape” with a large specific surface area more effectively exhibits these characteristics, and a wide range of possibilities can therefore be expected when it is used as a functional material.
To fabricate a material into a particle shape, two methods are typically considered: the top-down method of grinding from the bulk material, and the bottom-up method of accumulation from the molecular level. For chitosan, the top-down fabrication approach is simple and has been investigated extensively. However, it is preferable to use a bottom-up procedure to control the shape better. In general, when using the bottom-up method, it is necessary to dissolve or disperse the material to the molecular level in an appropriate solvent. Chitosan itself, like cellulose, dissolves sparingly in water and organic solvents due to the hydrogen bonds between molecular chains, but it can be dissolved in acidic aqueous solutions by protonation of the amino groups. Taking advantage of this property, our group added chitosan to an acidic aqueous solution, from which it was formed into a particle shape.
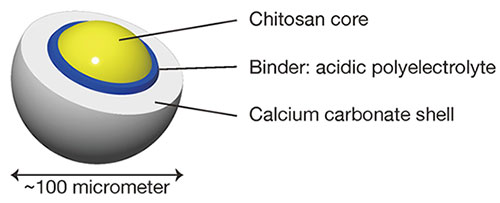
Another technique for adding more value to chitosan is to make it into a composite material by combination with other substances. Composites that have been studied so far include combinations with metals and other materials, and there are also examples of combinations with silver nanoparticles that are being studied in anticipation of their antimicrobial properties4. In our group, we are primarily studying combinations with calcium carbonate: a naturally occurring calcium salt that, together with chitin and chitosan, is a component of the exoskeletons of crustaceans. Therefore, the resulting composite material can be called an organic-inorganic hybrid material that is inspired by natural materials. Many composites of organic and inorganic materials are simple mixtures, and the distribution of the components is not controlled. In our proposal, the organic and inorganic components are distributed in controlled positions and shapes, a key point of difference from other research efforts5-7. If the distribution of organic and inorganic components within a material can be controlled, the desired functionality can be achieved. The organic and inorganic components in this study are arranged inside and outside, respectively, and combined with the particle shape, the system is designed to be a core-shell type (Fig. 2). Specifically, the inner core is made of chitosan and the outer shell is made of calcium carbonate. In this paper, I will introduce the process our group has adopted for making these particles, and report on the investigations of their application as drug carriers.
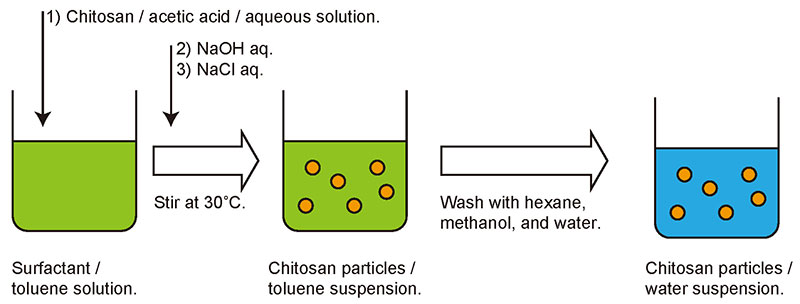
As the details of the particle fabrication process have been described in a previous report7, only a brief outline is presented here. Chitosan particles were prepared in excess toluene in which the surfactant sorbitan monolaurate had been dissolved. First, chitosan was dissolved in acetic acid to make an acidic aqueous solution. A small amount of chitosan solution was then added to the toluene and the mixture stirred vigorously. Because there is sufficient surfactant in the toluene, the aqueous chitosan solution disperses as micro-droplets. When an aqueous solution of sodium hydroxide was added to the mixture and stirred continuously, the acidic acetic acid solution in which the chitosan was added was neutralized, and the chitosan precipitated as a solid in droplet form. Sodium chloride solution was then added and the mixture was further stirred to obtain a suspension of chitosan particles (Fig. 3). The suspension was washed with an organic solvent and water, and finally the particles were recovered by lyophilization.
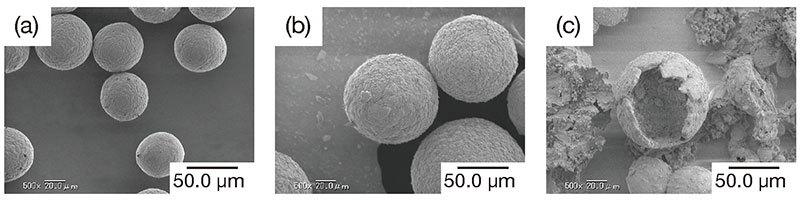
Scanning electron microscopic (SEM) images of the chitosan particles obtained are shown in Fig. 5 (a). The chitosan particles were almost spherical, with a particle diameter of about 40 micrometers. We believe that the surfactant stabilizes the w/o emulsion in toluene, and the chitosan in the aqueous droplets is precipitated as a solid without any change in shape and fixed into spherical particles.
The coating of calcium carbonate on the surface of chitosan particles was achieved by a process inspired by biomineralization. The minerals that organisms produce inside or outside the body are called biominerals, and the production process is known as biomineralization8. Examples of biominerals include pearl9, shells, bones, and the exoskeletons of crustaceans, many of which are found in the hard tissues of small marine animals. Biomineralization is a spontaneous accumulation and precipitation of inorganic salts, and has recently attracted attention as having a low environmental impact and for being an energy-saving process.
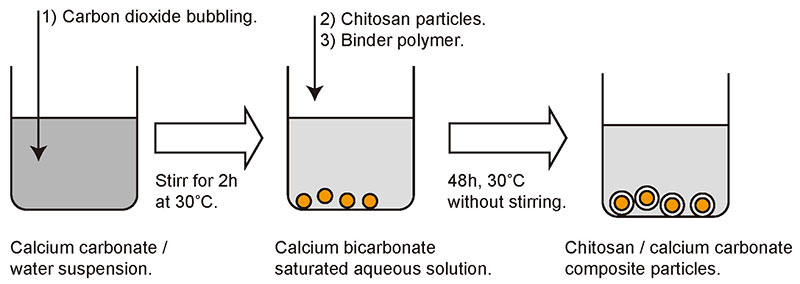
Our group mainly uses calcium carbonate, which is accumulated as a shell on the surface of chitosan particles by spontaneous precipitation. The procedure is shown in Fig. 4. First, an aqueous solution of calcium carbonate is prepared. Since calcium carbonate is sparingly soluble in water, carbon dioxide is bubbled through a suspension of calcium carbonate to produce a solution in the form of calcium bicarbonate. All that is required then is to mix the prepared chitosan particles and the polyelectrolyte that acts as an auxiliary agent for precipitation into the aqueous solution and allow it to stand for 48 hours. Polyelectrolytes such as polyacrylic acid and polyglutamic acid have a negative charge in aqueous solution and are believed to play a role in mediating between the positively charged chitosan surface and calcium ions . The most important feature of this procedure is that it does not require agitation of the liquid and proceeds simply while the mixture is standing.
SEM images of the composite particles obtained are shown in Fig. 5 (b). Like the chitosan particles used as the core, they were spherical, and only the average particle diameter was greater. From these results, we could infer that a uniformly thick calcium carbonate layer was deposited on the surface of the chitosan particles. In support of this contention, a photograph of collapsed particles is shown in Fig. 5 (c), which also reveals the presence of a thick shell.
Next, the surface was analyzed by attenuated total reflection / Fourier-transform infrared (ATR-FTIR) spectroscopy to verify that the shell was indeed composed of calcium carbonate. The ATR technique only extracts information to a depth within a few micrometers from the surface. It showed that there was almost no chitosan near the particle surface, and that at least the particle surface to a depth of several micrometers was composed solely of calcium carbonate. These findings imply the existence of a core-shell structure.
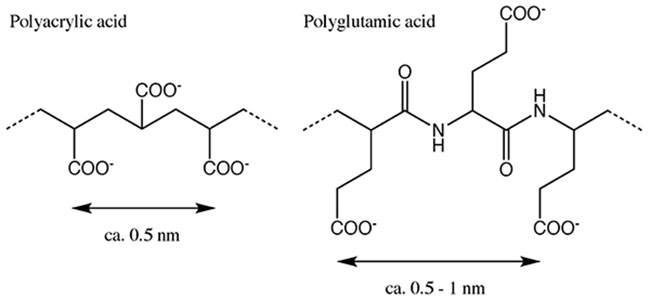
We confirmed that the surface of the composite particle was covered with a thick layer of calcium carbonate, but the actual structure was unknown. The crystal structure was thus investigated using X-ray diffraction (XRD), which revealed that the crystal structure of calcium carbonate was that of calcite when polyacrylic acid was used as a binder, and vaterite when polyglutamic acid was used as a binder7. Calcium carbonate is known to occur in three common crystal forms: calcite, aragonite and vaterite. The most stable of these structures is calcite, while aragonite and vaterite are less stable8. In our method, we were able to control the crystal form of the precipitated calcium carbonate by changing the type of binder polymer added. This can be explained by hypothesizing that the binder polymer plays a role in matching the positive charge between the chitosan surface and the calcium ions. The spacing of the carboxyl groups of polyacrylic acid, which played a role in the formation of calcite, was approximately 0.5 nm, which is almost identical to the interionic distance of calcium ions (0.499 nm) in the (001) face of the calcite crystal, and it appears that crystal formation was induced in the above process of calcium carbonate accumulation (Fig. 6)11,12. For polyglutamic acid, however, the alkyl chain between the carboxyl group and the main chain may prevent the distance between the carboxyl groups from being constant, resulting in the formation of a crystal structure other than that of calcite.
Our organic-inorganic composite particles had a controlled structure with crystalline calcium carbonate on the exterior and chitosan core particles in the interior. We therefore decided to study the potential use of this system as a microcapsule. Because the interior is an organic phase, organic compounds such as drugs can be impregnated or loaded into the core. An outer shell composed of inorganic crystals can be expected to serve as a protective layer for the inner material. Hence, in this research, a model drug was encapsulated in our core-shell composite microparticle to investigate its applicability as a drug carrier.
Lumichrome, a photodegradation product of riboflavin (vitamin B2), was selected as the model drug. As riboflavin is readily photodegraded, the experiment was conducted using a pre-degraded product. Lumichrome was dissolved together with chitosan when preparing the acetic acid solution, and the same particle-formation procedure as before was used to produce a calcium carbonate shell, yielding particles that contained lumichrome. The shape and size of the particles obtained by this method were not changed by the inclusion of lumichrome.
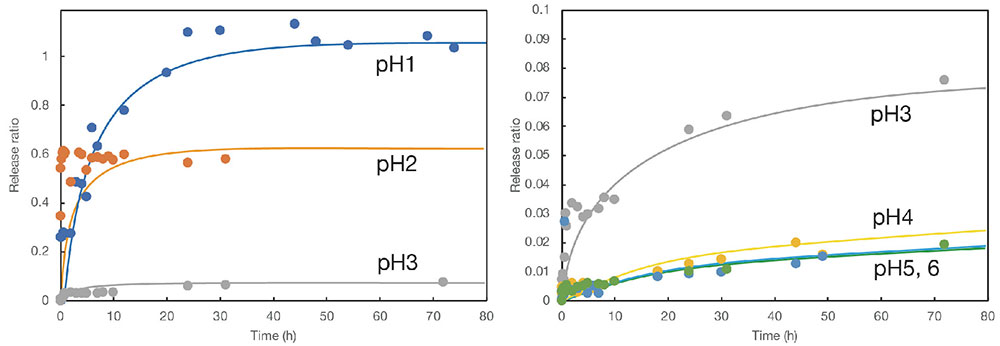
Next, these lumichrome-containing core-shell particles were immersed in a buffer solution prepared at selected pH values, and the release behavior of the model drug was evaluated by measuring the fluorescence of the supernatant aqueous solution over time. As lumichrome is a fluorescent substance, even trace amounts of its emission can be detected by fluorometry. Examples of the results are shown in Fig. 7. The vertical axis shows the release ratio for lumichrome from the particles and the horizontal axis the immersion time in the buffer solution. The results reveal that the release ratio of lumichrome increases with time and that its release behavior changes with pH value. However, as the initial slopes in the graph are relatively steep, the release behavior cannot be described as sustained. Fig. 7 shows the results for core-shell particles with calcite shells (prepared by adding polyacrylic acid as a binder). The system with vaterite shells showed similar behavior, although the initial release was slightly faster.
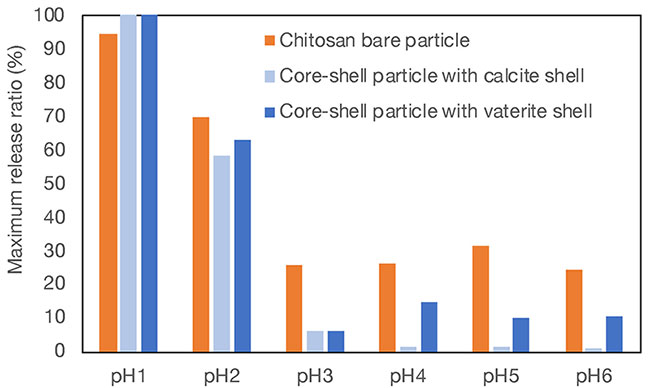
The release ratio after 48 hours, when the line was almost flat, was designated as the maximum release ratio, and the maximum release ratios at different pH values were determined (Fig. 8). The graph presents results for chitosan particles without shells (bare particles) and for two types of core-shell particles with different crystal structures. It was found that the drug release ratio from particles with shells was lower than that from bare particles in the range at pH values of 3 and above. This indicates that the presence of calcium carbonate protects the interior in the weakly acidic region. On the other hand, in the strongly acidic region of pH 1 and 2, the presence or absence of the shell did not substantially affect the release ratio. This reflects the fact that calcium carbonate dissolves rapidly in an acidic environment. Interestingly, in the near-neutral region at pH values of 4 and above, there was a difference in the release from core-shell particles with two different crystal structures. When the crystal form was calcite, the release ratio was lower than that when the crystal form was vaterite. The more stable crystalline form of calcite, as compared with vaterite, may be able to protect the core more effectively in a weakly acidic environment.
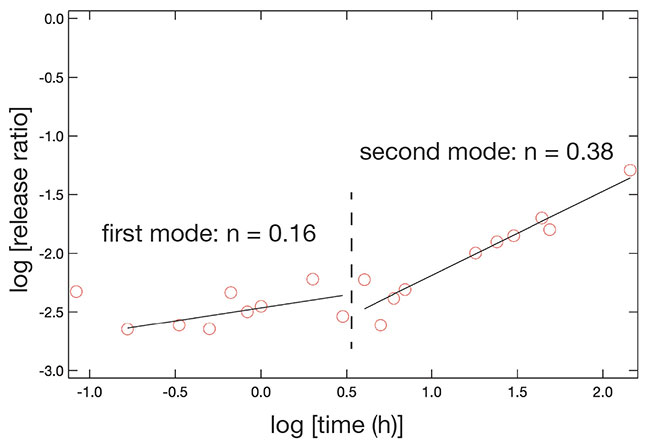
Next, we analyzed the changes in release behavior over time from a kinetic perspective, using a Korsmeyer-Peppas plot, an analytical method used to model drug release from formulations13. This technique involves fitting data for the release from a matrix drug carrier to a power function and inferring the type of diffusion from its exponent multiplier. The logarithm of the amount of emission is plotted on the vertical axis and the logarithm of time on the horizontal axis, and the slope is calculated after approximation to a linear function. A sample plot analyzing the drug release behavior from core-shell particles with a calcite shell at pH 4 is shown in Fig. 9. It was not possible to approximate the data with a single line, but it was judged appropriate to use two lines. Both slope values indicate that the release behavior follows Fick’s law of diffusion, but it is also clear that there are two modes of drug release behavior. Combining this finding with the fact that the particles have a core-shell structure, the first half of the slope appears to be influenced by the shell, while the second half reflects the release from the chitosan core particles. This is supported by the fact that the slope of the second half of the line of approximation is close to the slope obtained for the release from bare chitosan core particles without a shell. The slope of the plot corresponding to the first mode was much smaller than that of the second mode, and this analysis clearly indicated that the presence of calcium carbonate shell, the outer component of the core-shell structure, affected the release of the drug.
In this paper, I have described a method for controlling the shape and structure of organic-inorganic core-shell composite microparticles, with which our group is seeking to utilize the marine biomass chitosan as a functional microparticle material. This bottom-up approach to particle shape and combination with calcium salts inspired by biomineralization is a viable development process, and we hope to extend our investigations systematically and strategically. In addition to the research introduced in this paper, our group has also been working on particle formation, composite formation, and structural control on a millimeter scale, and we plan to demonstrate the potential of chitosan as a material in the future. The material chitosan (and chitin) has a variety of functions other than those introduced in this article, and is a constant source of interest for our group. Although cellulose is currently the only biomass that can be used in large quantities, it is hoped that research on chitosan will further continue as a biomass candidate with higher functionality.
This work was supported by JSPS Grants-in-Aid for Scientific Research JP21K12314.