
Shoutaro Tsuji
Faculty of Medical Technology & Clinical Engineering, Gunma University of Health and Welfare
Dr. Tsuji graduated from the Faculty of Pharmaceutical Sciences, Hokkaido University, in 1991. In 1996, he completed his doctoral course and obtained a PhD in pharmaceutical sciences from the Graduate School of Pharmaceutical Sciences, Hokkaido University. Thereafter, he served as an assistant professor at the School of Pharmaceutical Sciences in the University of Shizuoka, as a postdoctoral researcher at the Research Institute in Osaka Medical Center for Cancer and Cardiovascular Diseases, as an assistant professor at the Research Institute for Microbial Diseases in Osaka University, as a postdoctoral researcher at Florida Atlantic University, and as a principal investigator at the Kanagawa Cancer Center Research Institute. Since around 2010, when he arrived at his position at the Kanagawa Cancer Center Research Institute, he has been engaged in R&D for antibody drugs focusing on mesothelioma. He has been in his current position since 2021.
The use of antibody drugs has been spreading rapidly because of their excellent pharmacological effects and the recent advances in antibody humanization technology and biologic production technology. Currently, a large number of antibody drugs are being marketed as block busters; as such, antibody drugs may be described as occupying an established position in the pharmaceutical industry. On the other hand, suitable targets for disease-specific treatments are increasingly being depleted; a key step in antibody drug development is the discovery of new-generation target antigens.
We are working on discovering specific cancer antigens for malignant mesothelioma, an intractable cancer. Mesothelioma has long been an “indistinct” cancer as it lacks good cancer markers and hence is quite difficult to diagnose pathologically. Recognizing a new protein antigen encompassing a “glycopeptide region with mesothelioma-specific glycosylation,” our anti-mesothelioma antibody is highly promising as a new antibody drug seed expected to contribute to the diagnosis and treatment of mesothelioma, and as a drug discovery seed for targeting glycans. This article provides a historical overview of the research and development, and future prospects for clinical application of this antibody.
It has long been known that cell glycosylation is altered by cancer transformation. To detect a mucin-like membrane protein resulting from altered glycosylation, in particular, is widely used as a tumor marker test in clinical settings1,2, and “cancer glycan antigens” are attracting attention as new-generation target antigens to resolve the issue of antibody drug target depletion3. On the other hand, the glycan changes in tumors are mainly glycan structure simplifications or glycan addition site changes; the glycan structure is seldom tumor-specific4. It has been increasing evident that an antibody cannot be tumor-specific unless it recognizes a conformational change in the core protein due to glycosylation in its vicinity, rather than the glycan as such5 or unless it simultaneously recognizes the changed glycan and peptide6.
However, only a few antibodies have been reported to specifically recognize such glycan-bound peptides, and how the antibody recognizes them has been reported in even fewer cases. An antibody capable of recognizing “tumor-specific glycosylation” on a “tissue-specific protein antigen” has the potential to be an excellent cancer-cell-specific antibody drug; however, it remains unclear whether any disease-specific antibody exists that simultaneously recognizes a glycan and peptide.
The malignant mesothelioma, which we have targeted for our pharmaceutical development, is a treatment-resistant (intractable) cancer caused by past inhalation of asbestos fibers, and it has recently emerged as a major asbestos-related disease posing a social issue of heath damage. The molecular pathological diagnosis of mesothelioma is difficult because of the scantiness of genetic variations and characteristic gene expressions, thus necessitating diagnosis by immunohistological staining with more than one antibody7. However, because even well experienced experts often find it difficult to diagnose with the use of more than one antibody, there is a strong demand for cancer antigen-based tests of excellent specificity and sensitivity for mesothelioma.
We considered it difficult to find a cancer antigen of high specificity for mesothelioma by simple gene expression level analysis, and began searching for a mesothelioma cancer antigen by preparing a monoclonal antibody to mesothelioma-specific post-translational modifications (particularly glycosylation). As a result, we developed an anti-mesothelioma antibody, SKM9-2, that exhibits much higher sensitivity and specificity for mesothelioma than conventional markers and recognizes sialylated protein antigens, and succeeded in identifying HEG1 as an unknown mucin-like membrane protein antigen8.
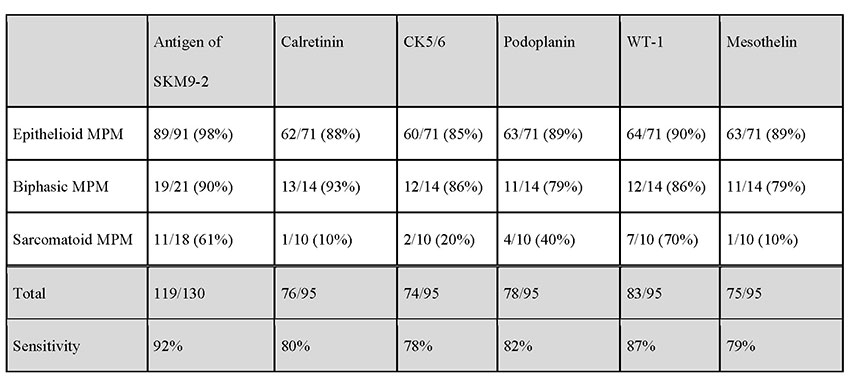
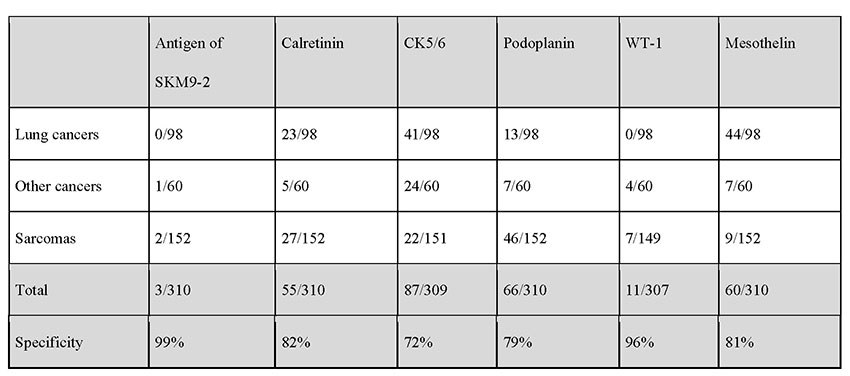
The positivity rate for SKM9-2 is up to 98% in epithelial mesotheliomas, which account for many cases of mesotheliomas. More than half of patients with sarcomatous mesothelioma, which is difficult to detect by pathologic diagnosis using conventional markers, test positive for SKM9-2, demonstrating a higher order of sensitivity than obtainable with the use of conventional mesothelioma markers (Table 1). Furthermore, SKM9-2 exhibits an extremely high specificity of 99% for mesothelioma, and little binding to any other type of cancer (Table 2) or normal tissue (Figure 1)8. Because the specificity levels of conventional mesothelioma markers are up to 70% to 80%, SKM9-2 is evidently superior to conventional markers for the pathologic diagnosis of mesothelioma and is expected to contribute to improving the diagnostic accuracy for mesothelioma9-11. Because SKM9-2 can be used in immunohistological staining of formalin-fixed paraffin-embedded sections, it is now commercially available as an immunohistochemical staining reagent for pathologic diagnosis from Nichirei Biosciences Inc., and it is already being marketed outside Japan by Bio SB, Inc.
 MPM, malignant pleural mesothelioma
MPM, malignant pleural mesothelioma
SKM9-2 recognizes high-molecular-weight cell surface antigens (400 kDa or more). Its analysis via glycosidase treatment suggests that SKM9-2 recognizes a specific region of a membrane protein that is modified by sialylated O-type glycan. We proceeded to purify it by means of acid-based fractional precipitation and gel filtration and other chromatographic techniques, and succeeded in identifying the antigen of SKM9-2. The identified molecular, HEG1, is a type I membrane protein consisting of a Ser/Thr-rich domain (accounting for 70% of the molecular structure) with many bound glycans and an EGF domains. Thus, its molecular structure is like that of membranous mucin8 (Figure 2). HEG1 was an unknown molecular species about which little had been reported and for which no effective commercially available antibody exists.
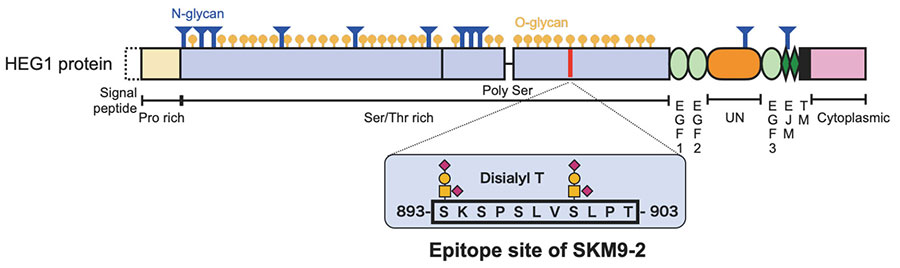
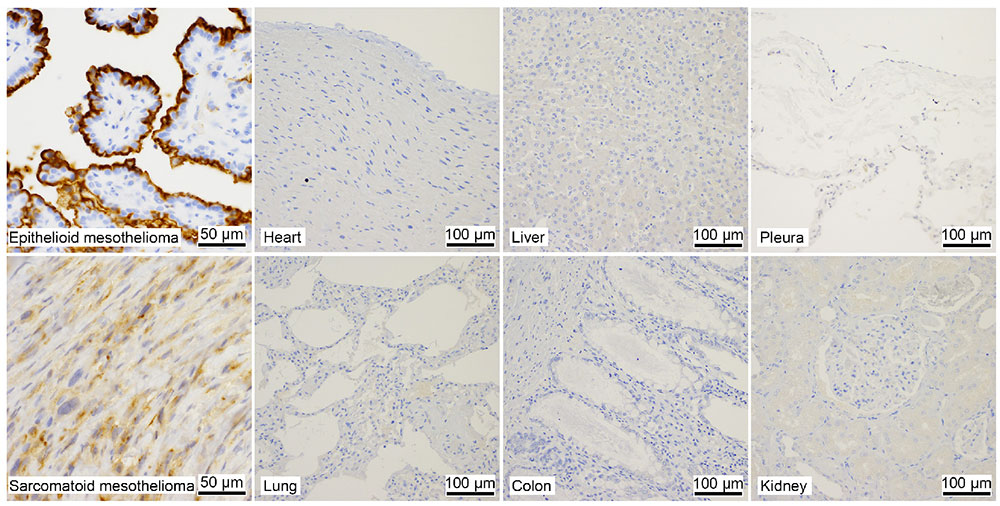
Immunostaining shows that SKM9-2 binds specifically to mesothelioma and does not bind to most organs, including the lung and heart, although HEG1 mRNA is detected in the lung and heart (Figure 1)8. In addition, the binding of SKM9-2 is dependent on the existence of sialic acid; therefore, it was considered that sialylated glycan modification occurred in the HEG1 of mesothelioma, which is not observed in the HEG1 of any other tissue, and that SKM9-2 recognized this glycosylated region of HEG1. Hence, we prepared an HEG1-deficient mutant by deleting portions of the N-terminus amino acid sequence in a stepwise fashion, and analyzed the epitope of SKM9-2 to determine the minimum peptide sequence 893-SKSPSLVSLPT-903. We furthermore analyzed the glycan structures in this sequence and determined the glycan-containing epitope structure12 (Figure 2).
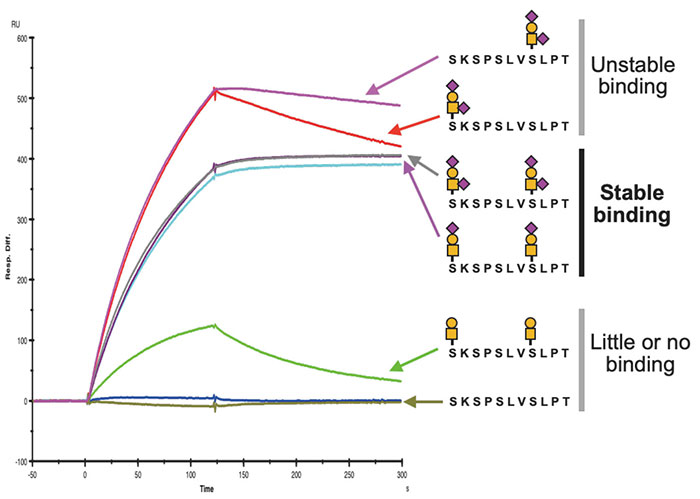
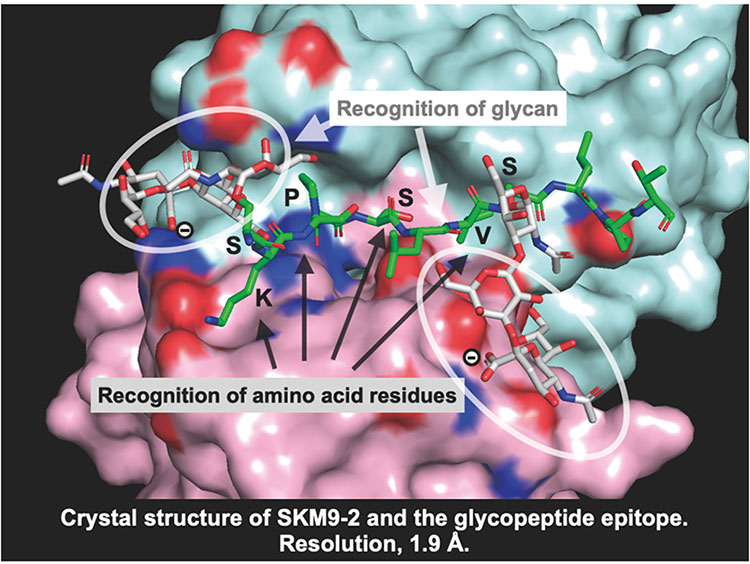
Based on the determined epitope structure, we synthesized a glycopeptide and performed surface plasmon resonance (SPR) analysis to determine its binding ability, with analysis showing that stable antibody binding requires the addition of two sialyl T or disialyl T glycans to 893Ser and 900Ser (Figure 3). X-ray crystal structure analysis found a deep groove between the H- and L-chains of the antibody, showing that the peptide moiety becomes engaged in the groove, and the antibody recognizes the epitope with the thus-added sialylated glycan wrapped by the H- and L-chains from both sides (Figure 4). These results indicated that SKM9-2 is a unique antibody that simultaneously recognizes the two glycans and a peptide.
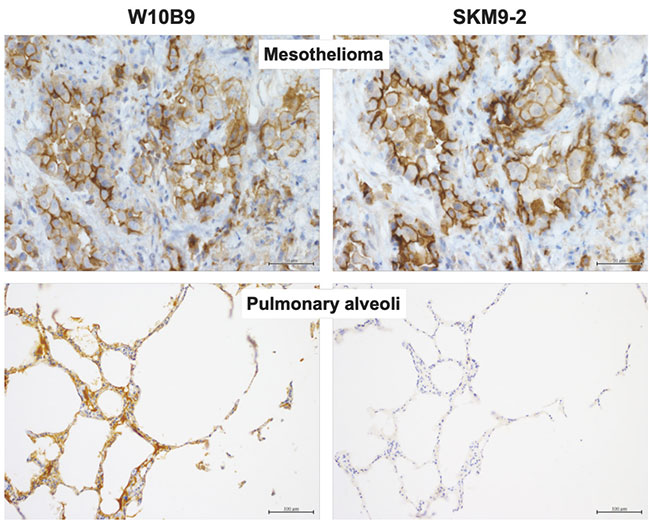
Since glycan recognition was found to be important, we prepared a non-glycan-dependent anti-HEG1 antibody (W10B9) and analyzed it to determine whether HEG1 non-reactive with SKM9-2 (HEG1 lacking sialylated glycan) is present in non-mesothelioma tissue. Some tissues such as pulmonary alveoli showed W10B9-positive, SKM9-2-negative staining patterns (Figure 5), suggesting the expression of HEG1 with no sialylated glycan added to 893Ser and 900Ser in such tissues. Therefore, the high specificity of SKM9-2 for mesothelioma was attributable to the recognition of a glycosylation characteristic of the HEG1 in mesothelioma.
SKM9-2 binding to major organs and healthy tissues is low, but highly specific for mesothelioma. With this feature, SKM9-2 was considered a promising seed for therapeutic antibody drugs for mesothelioma. Currently marketed antibody drugs include “traditional” antibody drugs that act by antigen neutralization or antibody-dependent cellular cytotoxicity (ADCC); “new-generation” antibody drugs, including labeled cytotoxic agents such as antibody-drug conjugates (ADCs)13 and antibody-radionuclide conjugates (ARCs)14, and bispecific antibodies15; and chimeric antigen receptor T (CAR-T) cytotherapy consisting of an antibody-TCR fusion gene incorporated in T cells16. Since SKM9-2 was not found to have pharmacological activities as a traditional antibody drug, we are now working on developing it as a new-generation antibody drug.
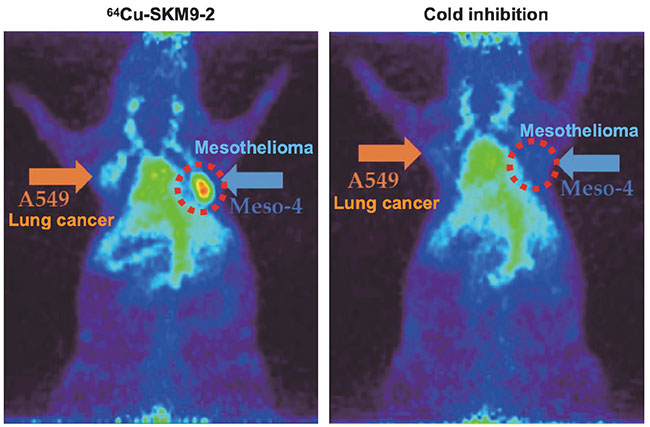
We administered radiolabeled SKM9-2 to a mouse model of subcutaneously transplanted mesothelioma, and found specific accumulation of SKM9-2 in mesothelioma tumors (Figure 6). An observation of SKM9-2 labeled with a pigment that emits fluorescence when taken up in cells showed that SKM9-2 bound to HEG1 on the cell surface is taken up into cells, suggesting its potential for applications to ADCs and ARCs. At present, we are working to create a master cell bank for the manufacture of humanized SKM9-2 and manufacture antibody drug substances, and planning to conduct a nonclinical study of these antibody drugs as ARCs.
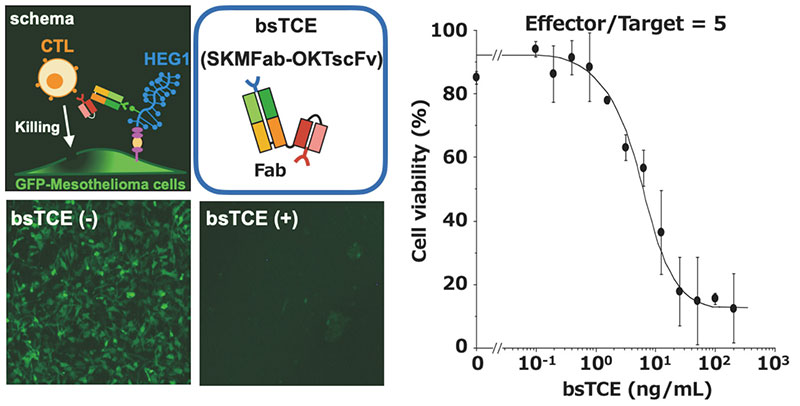
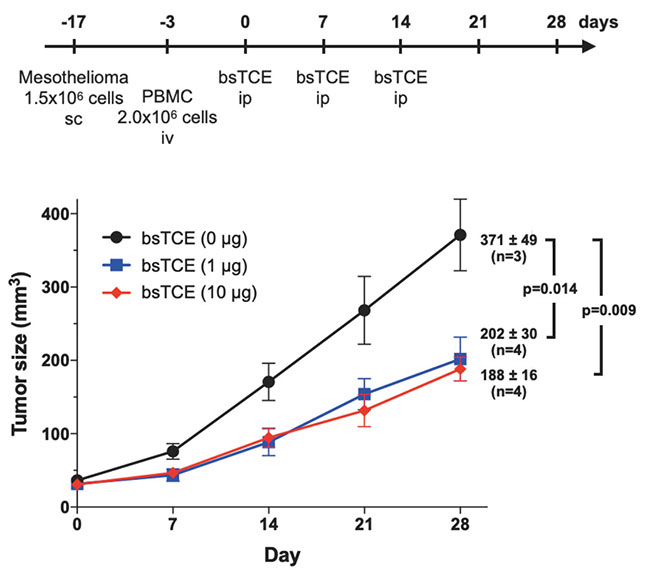
We are also developing a T cell-engaging bispecific antibody (bsTCE) involved in cellular immunity14. A representative of bsTCE is the tandem scFv type (BiTE), which is already approved for medical use, and which comprises two scFv units joined together. However, the stability of SKM9-2 scFv is poor, and its production in CHO cells is also poor. Therefore, we switched our focus to developing the bsTCE of the Fab-scFv type, which comprises an Fab of SKM9-2 conjugated with an scFv of anti-CD3 antibody, and investigated its performance. We found that bsTCE, when added to human peripheral blood mononuclear cells (PBMCs) and a target mesothelioma cell line, had a cytotoxic activity at a concentration of 100 ng/mL in all tested target cell lines (Figure 7). In addition, when transplanting a mesothelioma cell line subcutaneously to immunodeficient mice and then injecting bsTCE and human PBMCs, we found that tumor growth was suppressed significantly (Figure 8). Hence, bsTCE in combination with SKM9-2 possesses extremely potent anti-tumor activity against mesothelioma and is expected to be a highly promising candidate drug for mesothelioma therapeutics.
New-generation antibody drugs such as ARCs and bsTCE possess potent anti-tumor activity. In the process of their development, it is more important to verify their cancer specificity (lack of binding ability to healthy tissues) than that of conventional antibody drugs. However, in the case of disease-specific glycosylated antigens, their expression in individual tissues cannot be verified by mRNA-based expression analysis. Thus, their target specificity and safety are not easy to demonstrate. An immunohistochemical analysis suggests that the amount of glycosylated epitopes of SKM9-2 is small in healthy tissues. However, because the sections used for the immunohistochemical staining represent only a very small portion of the whole organ, and the mRNA of HEG1 is expressed in various organs, safety concerns by the licensing company remain and are unlikely to be eliminated. These safety and disposition issues are common problems in the development of drugs that target glycopeptides for which expression levels of the mRNA are disproportionate to amounts of the glycosylated target (epitopes). The only solution to this problem is to conduct a microdose study of a radiolabeled antibody in patients to examine the postdose tissue distribution of the antibody. However, such a first-in-human study would need to overcome high regulation, cost, and implementation hurdles. While glycan-related antigens are highly promising for new-generation disease targets as they are expected to show high specificity for target diseases, their licensing-out necessitates a first-in-human study; from the viewpoint of academia, the “valley of death” in drug development seems to be deeper with glycan-related antigens than with conventional protein antigens.
Fortunately, the present study was started in collaboration with a radiopharmaceutical firm with the support of the Japan Agency for Medical Research and Development (AMED); the initiation of a first-in-human study has become feasible, and a nonclinical study has been embodied. If SKM9-2 is brought to practical application, it would be a model for the future development of glycan-related antibody drugs. Aiming to deliver a drug to patients with mesothelioma as soon as possible, we are endeavoring to facilitate drug development for early market launch.
The author thanks the many co-researchers for their cooperation and the AMED for their financial support (Grant Number JP16cm0106406, JP18ae0101071).