
Yusuke Yamashita
Doctoral course student, Graduate School of Science and Engineering, Kagoshima University
His research has focused on the development of novel biomaterials.

Takayuki Takei
Professor, Graduate School of Science and Engineering, Kagoshima University
He received his Ph. D. (Engineering) from Kyushu University in 2007. He was a research fellow (PD) of the JSPS (Japan Society for the Promotion of Science) in 2007. In 2008-2011, he was an assistant professor in Graduate School of Engineering, Kyushu University. He was an associate professor in Graduate School of Science and Engineering, Kagoshima University in 2012-2019, and promoted to Professor in 2020. He received the Award for Outstanding Young Researcher from The SCEJ (Society of Chemical Engineers, Japan) in 2014. His research has focused on the development of novel biomaterials based on interfacial science.
Chitosan is widely known to accelerate wound healing. Therefore, various wound dressings based on chitosan have been reported. Although hydrogel-type wound dressings are suitable for moist wound healing, it is difficult to develop hydrogel-type chitosan wound dressings due to two reasons. When we prepare chitosan hydrogels, chitosan first needs to be dissolved in water. However, since chitosan dissolves only in acidic water, the resulting hydrogel is also acidic, making it unsuitable for application to wounds. In addition, the polymer dissolved in an aqueous solution needs to be cross-linked with chemical cross-linkers to form hydrogels. However, because chemical cross-linkers are highly toxic, hydrogels containing them are not suitable for medical applications. Here, we describe our chitosan hydrogels exhibiting neutral pH and containing no additives, which are prepared by freeze-thawing or autoclaving chitosan aqueous solutions without using chemical cross-linkers.
Moist would healing is known to promote wound healing by providing an optical moist condition to wounds1. Wound dressings are medical products designed to cover wounds and keep them in moist environment. Hydrogels are suitable as wound dressings due to their hyperhydrous structure. Many researches have been conducted to develop hydrogel-type wound dressings with high performances.
Chitosan has an inherent biological activity to accelerate wound healing. Therefore, various wound dressings based on chitosan have been reported. Although hydrogel-type wound dressings are suitable for moist wound healing, it is difficult to develop hydrogel-type chitosan wound dressings due to two reasons. When we prepare chitosan hydrogels, chitosan first needs to be dissolved in water. However, since chitosan dissolves only in acidic water, the resulting hydrogel is also acidic, making it unsuitable for application to wounds2. In addition, the polymer dissolved in an aqueous solution needs to be cross-linked with chemical cross-linkers to form hydrogels. However, because chemical cross-linkers are highly toxic, hydrogels containing them are not suitable for medical applications3. Here, we describe our chitosan hydrogels exhibiting neutral pH and containing no additives, which are prepared by freeze-thawing or autoclaving chitosan aqueous solutions without using chemical cross-linkers.
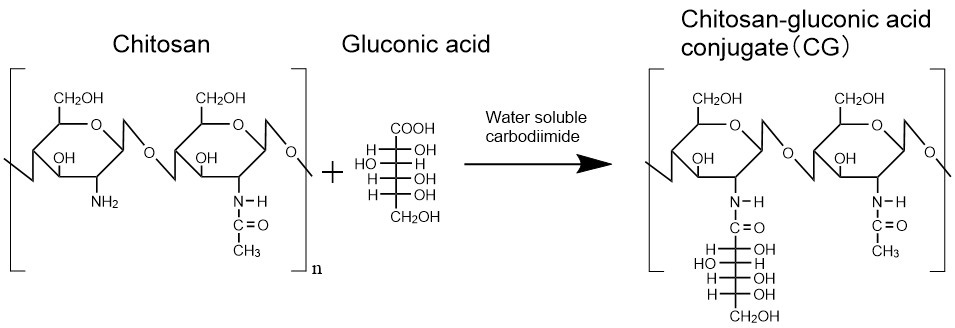
Chitosan has been reported to have water-insoluble rigid crystal structure. Therefore, to obtain a chitosan aqueous solution, the solid polymer needs to be added to an acidic aqueous solution to achieve protonation of the amino groups and subsequent dissolution of the polymer2. We expected that the crystallization of chitosan could be sterically inhibited by modifying the amino group of chitosan with some molecules. As the molecule to be modified to chitosan, we used gluconic acid, which has high biological safety and is contained in various food products. Water-soluble carbodiimides was used to form carboxyl-amine conjugation between amino groups of chitosan and carboxyl groups of gluconic acid (Fig. 1). Contrary to expectations, the solid chitosan-gluconic acid conjugate (CG) hardly dissolved in neutral water when it was added to the water directly. On the other hand, once dissolved in an acidic solution, CG hardly precipitated even in neutral water prepared by gradually adding an alkaline solution to the polymer solution. We confirmed that unmodified chitosan precipitated before reaching neutral pH. These results indicate that the incorporated gluconic acid sterically inhibited the crystallization of chitosan. In addition, we found that CG precipitated in some samples of neutral CG aqueous solutions after they were stood for more than a few days, suggesting that the neutral CG aqueous solutions are thermodynamically unstable. Chitosan derivatives modified with other types of aldonic acid also had the similar solubility.
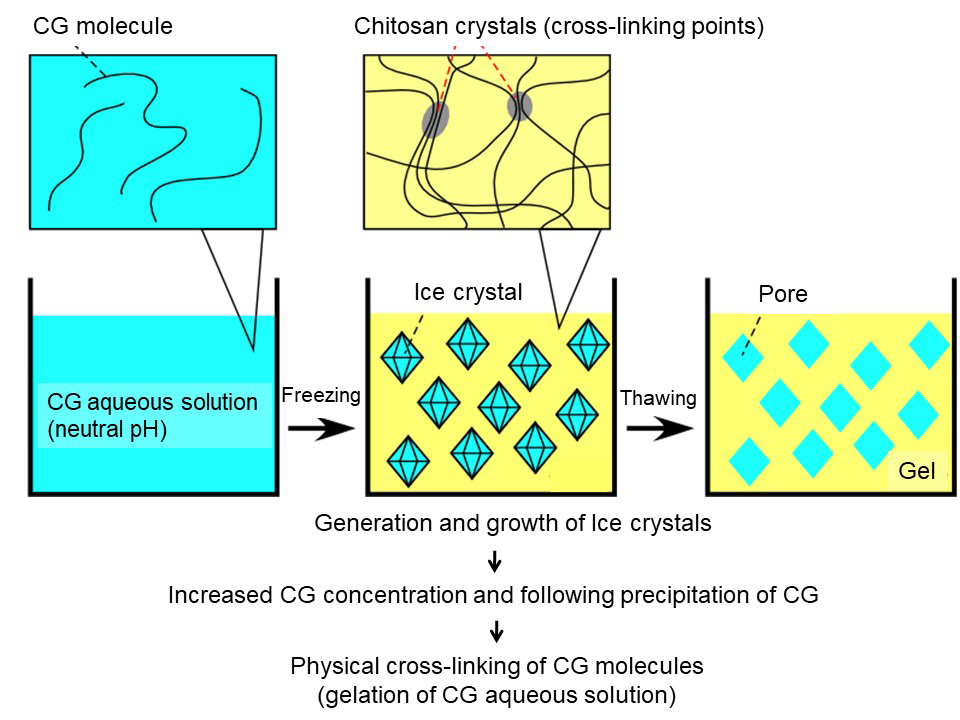
We showed that CG hydrogels could be formed by freezing at -30°C the neutral CG aqueous solutions prepared according to the procedure described above and subsequent thawing them at room temperature (Fig. 2). The possible mechanism of the gelation is as follows (Fig. 3). After the temperature of the neutral CG aqueous solutions drops below 0°C, ice crystals form in the solution and grow. Since the ice crystals hardly contain other molecules (e.g. salts and CG molecules), the concentration of CG dissolved in the water still remaining as a liquid phase increases. Eventually, the CG precipitates after the concentration reaches saturation solubility. In this state, the CG precipitate is formed around the ice crystals, resulting in formation of a spongy framework of CG hydrogel. The precipitated CG would be highly crystallized. After the frozen construct is stood at room temperature, the ice crystals thaw and return to water. The pH of the water is neutral as in the case before freezing. As described above, solid CG hardly dissolves directly in neutral water. Therefore, The sponge-like skeleton formed by solid CG would hardly dissolve in the neutral water after thawing, resulting in formation of CG hydrogels. Observation of the CG hydrogels under a microscope showed the presence of the sponge-like skeleton.

In many cases, the solubility of hydrophilic polymers in water increases with increase in temperature (temperature-dependent solubility). On the other hand, the solubility of chitosan hardly increases with increasing water temperature. However, as described above, the solubility depends on the pH of the aqueous solution. The pH-dependent solubility of CG suggests that the CG hydrogels prepared in freeze-thawing process are highly stable against heat. Therefore, we tried to sterilize the CG hydrogels by autoclaving. After autoclaving, the hydrogels not only retained their shape, but also shrank, suggesting enhanced mechanical strength of the hydrogels. In addition, as mentioned above, neutral CG aqueous solutions are thermodynamically unstable. From these findings, we expected that autoclave treatment of neutral CG aqueous solutions results in gelation of the solution. As expected, the autoclave treatment caused gelation of the CG aqueous solution (Fig. 4). Presumably, when a neutral aqueous CG solution is heated, the thermal motion of the CG molecules becomes more intense, which causes dehydration of the molecules and subsequent crystallization of chitosan (the physical cross-linking points of the molecules).

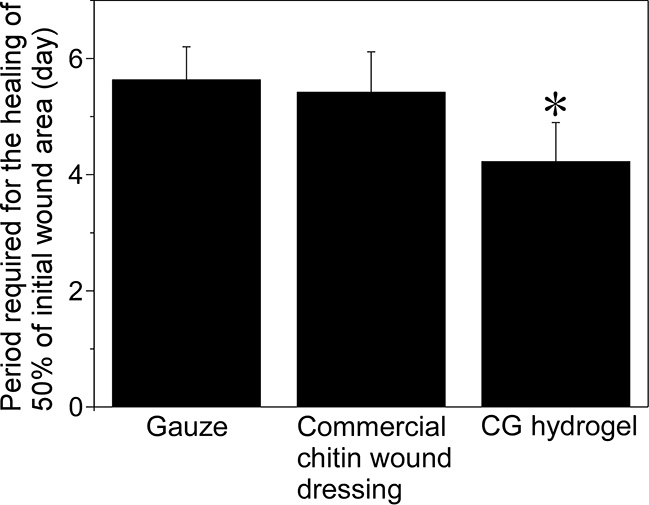
The chitosan hydrogels we developed have a great potential to promote wound healing through the synergistic effects of moist healing and the inherent biological activities of chitosan. Here, we examined the efficacy of CG hydrogels in repairing skin wounds in rats (Fig. 5). The hydrogels were applied to circular skin wounds. A commercial dry wound dressing made of chitin was used for comparison with CG hydrogels. The CG hydrogels took the shortest time for the healing of 50% of the initial wound area. Many neutrophils that secret proteins that promotes wound healing were observed on the wound surface in contact with the CG hydrogels. As expected, the reasons why CG gel promoted the wound healing would be the synergistic effect of moist healing and the wound healing-promoting effect of chitosan molecules.
In this paper, we described the preparation procedure, gelation mechanism, and various properties of the chitosan hydrogels we developed. The most important feature of the hydrogels is that they are neutral and contains no chemical cross-linking agents, making them suitable for medical applications. For commercialization of the CG hydrogels in medical market, we have proceeded optimization of properties of the hydrogels.