Shozo Jinno
Professor, Department of Anatomy and Neuroscience, Graduate School of Medical Sciences, Kyushu University
Shozo Jinno graduated from Kyushu University School of Medicine in 1992 and entered the Department of Psychiatry and Neurology at Kyushu University Hospital in the same year. He then received his PhD (Medicine) from the Graduate School of Medical Sciences, Kyushu University and became a research associate at the Department of Anatomy and Neurobiology, Graduate School of Medical Sciences, Kyushu University in 2000. In 2004, he became a postdoctoral researcher at MRCANU, Oxford University, UK, and returned to Japan in 2006. He was appointed to lecturer at the Department of Anatomy and Neurobiology, Graduate School of Medical Sciences, Kyushu University in 2008, associate professor at the Department of Anatomy and Neurobiology, Graduate School of Medical Sciences, Kyushu University in 2010, and professor at the Department of Anatomy and Neuroscience, Graduate School of Medical Sciences, Kyushu University in 2011, where he remains to this day.
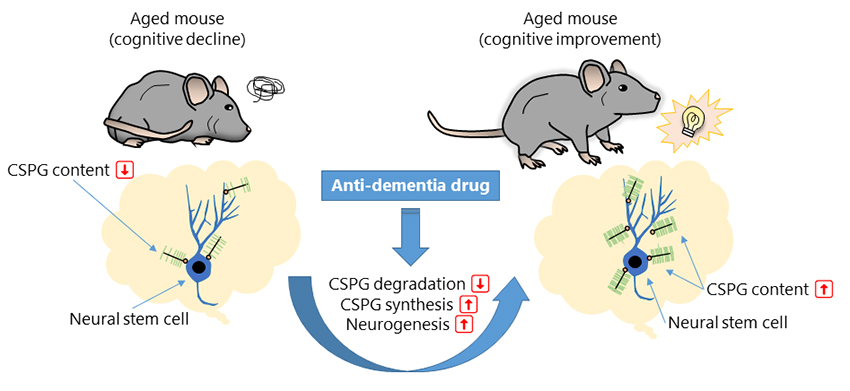
The extracellular matrix (ECM) plays a central role in brain homeostasis1. Earlier studies have shown that the ECM is involved in the control of neuronal and glial functions through regulation of growth factors, neurotrophic factors, and neurotransmitters. It has also been shown that ECM is involved in embryonic neurogenesis and axonal outgrowth during brain development2. Interestingly, recent studies have suggested that the ECM constitutes a microenvironment called the “neurogenic niche” in the dentate gyrus of the adult hippocampus, where the ECM may be involved in the proliferation and differentiation of neural stem cells (NSCs) and neuronal progenitor cells (NPCs) into newborn granule cells (NGCs) 3. We have reported that chondroitin sulfate proteoglycan (CSPG), one of the major molecules of the brain ECM, may improve cognitive function via promotion of adult neurogenesis in the hippocampus. This perspective outlines that CSPG may work as a molecular target for dementia treatment.
The hippocampus is the center of cognition and emotion4. Although neurogenesis in the brain normally ends by late childhood, the hippocampus is one of the few brain regions where neurons (granule cells) are produced, even in adults. NSCs give rise to NPCs, which then differentiate into NGCs in the dentate gyrus of the hippocampus. NGCs mature over approximately two months and integrate into the existing neuronal circuits in the hippocampus5. It has been suggested that NGCs play important roles in the regulation of cognitive processes, such as learning and memory6. Deficits in adult neurogenesis may underlie neurological and psychiatric disorders. In 2018, we have reported that CSPG in the dentate gyrus may be involved in the promotion of adult hippocampal neurogenesis by the “enriched environment” 8. Since then, the regulation of adult neurogenesis in the hippocampus by ECM has become a topic of neuroscience research9.
Memantine (MEM) is an open-channel blocker of the N-methyl-ᴅ-aspartate (NMDA) receptor and is used to treat moderate to severe Alzheimer's disease10. Although previous studies have suggested that MEM treatment may prevent cognitive decline by protecting neurons from oxidative stress and excitotoxicity11, the details remain unclear. Inspired by the reports showing that MEM inhibits the degradation of the cartilage ECM, which is composed of collagen II and aggrecan12, we aimed to understand the potential effects of MEM on the brain ECM and adult hippocampal neurogenesis.
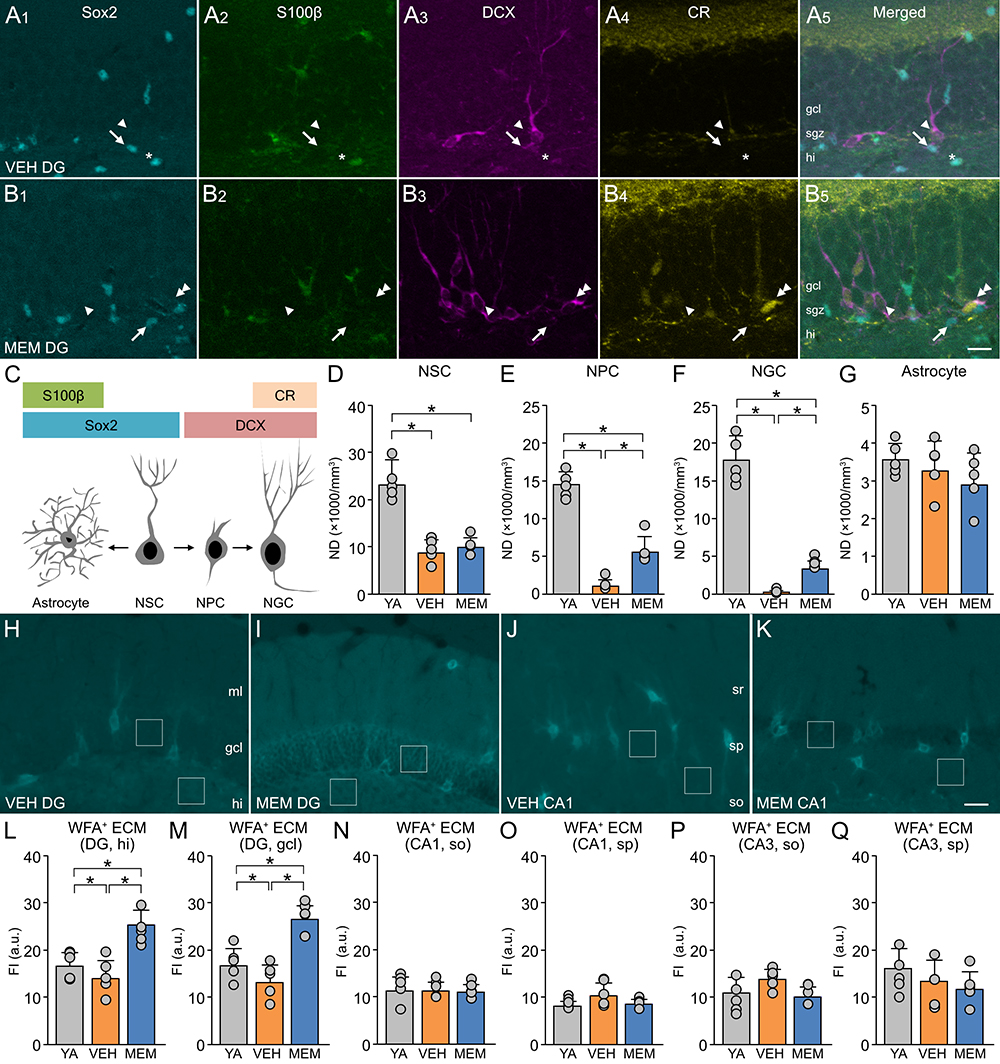
We investigated the potential effects of MEM on adult neurogenesis in the hippocampus. In our experiments, we identified neurogenesis-associated cells based on the expression patterns of molecular markers in the dentate gyrus of the hippocampus using molecular markers (Figure 1A-C). In middle-aged (MA) mice, staining for neurogenesis-related molecules was enhanced in the MEM-treated group compared to the vehicle (VEH)-treated control group. Next, we measured the numerical density (ND) by using the optical dissector method13 (Figure 1D-G). The NDs of NSCs, NPCs, and NGCs were lower in VEH-treated MA mice than in VEH-treated young adult (YA) mice. It should be noted that the NDs of NPCs and NGCs in MA mice were increased after MEM treatment. Neither aging nor MEM treatment affected the ND of astrocytes in the hippocampus.
To investigate the potential effects of aging and MEM on ECM in the hippocampus, we labeled CSPG using plant lectin (Wisteria floribunda agglutinin, WFA) and measured the fluorescence intensity (Figure 1H-K). In the dentate gyrus, the fluorescence intensity of WFA decreased with age and increased with MEM treatment (Figure 1L, M). However, in the CA1 and CA3 regions, no age-related or MEM-induced changes were observed in the fluorescence intensity of WFA (Figure 1N-Q). These results suggest that MEM may increase the expression of CSPG in the dentate gyrus and restore the production of NGCs that declines with aging.
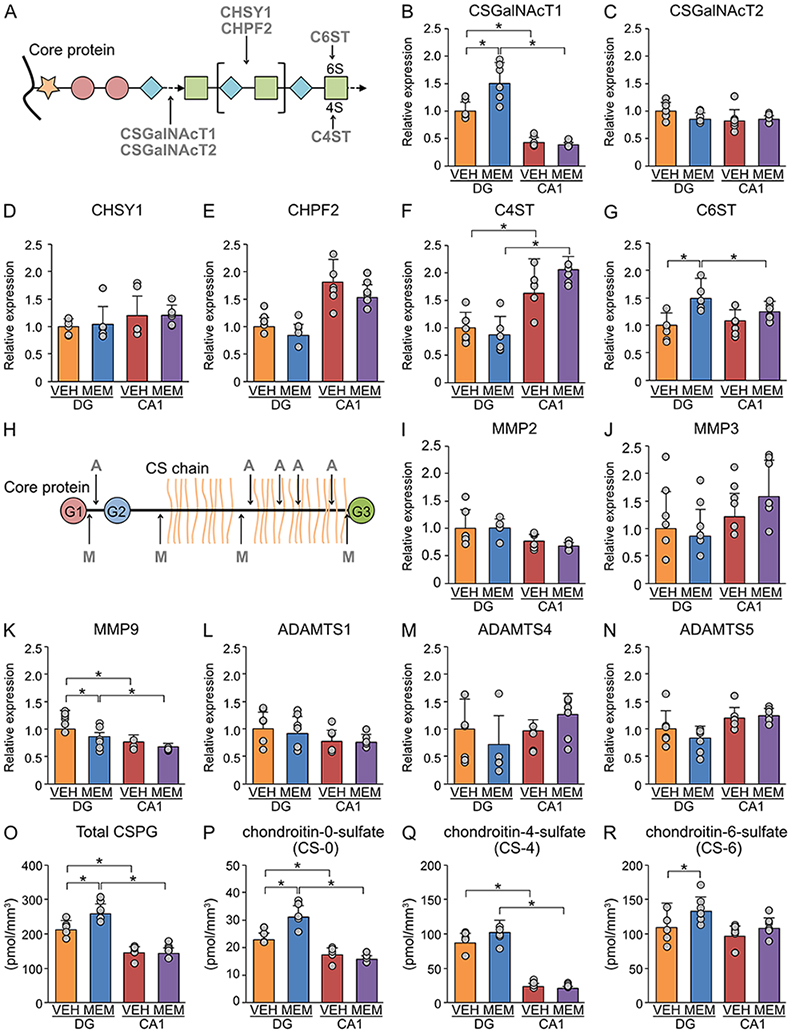
We examined the effects of MEM treatment on CSPG biosynthesis in the dentate gyrus of MA mice by reverse transcription-quantitative polymerase chain reaction (RT‒qPCR) (Figure 2A-G ). MEM treatment increased the expression level of chondroitin sulfate N-acetylgalactosaminyltransferase 1 (CSGalNAcT1), the rate-limiting enzyme for CSPG biosynthesis in the dentate gyrus. However, no changes were observed in the expression levels of CSGalNAcT2, chondroitin sulfate synthase 1 (CHSY1), and chondroitin polymerizing factor 2 (CHPF2) in the dentate gyrus. Similarly, MEM treatment did not affect the expression levels of chondroitin-4-sulfotransferase (C4ST), which is involved in the sulfation of CSPG, but increased the expression level of C6ST in the dentate gyrus. Meanwhile, in CA1 and CA3 regions, no expression changes were observed for these genes related to CSPG biosynthesis.
We also investigated the effects of MEM treatment on CSPG-degrading enzymes by RT‒qPCR (Figure 2H-N). The expression levels of matrix metalloproteinase 2 (MMP2), MMP3, a disintegrin and metalloproteinase with thrombospondin motifs 1 (ADAMTS1), ADAMTS 4, and ADAMTS5 were not altered by MEM treatment in either the dentate gyrus or Ammon’s horn. However, the expression levels of MMP9 in the dentate gyrus were suppressed by MEM treatment. Together, upregulation of CSPG biosynthesis and downregulation of CSPG-degrading enzymes by MEM treatment may increase CSPG expression in the dentate gyrus.
To confirm the above results, we measured the contents of CSPG by high-performance liquid chromatography (HPLC) (Figure 2O-R). The analysis showed that MEM treatment increased the contents of CSPG, chondroitin-0-sulfate (CS-0), and chondroitin-6-sulfate (CS-6) but not chondroitin-4-sulfate (CS-4), supporting the hypothesis that alterations in the expression of CSPG-related enzymes by MEM treatment may increase the contents of CSPG.
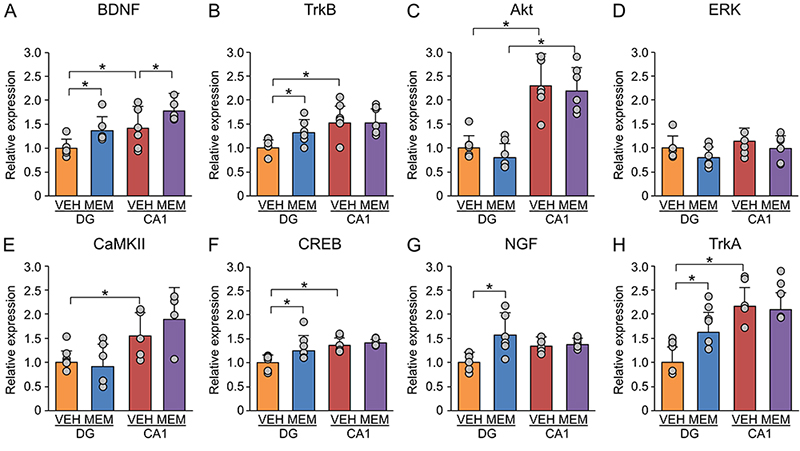
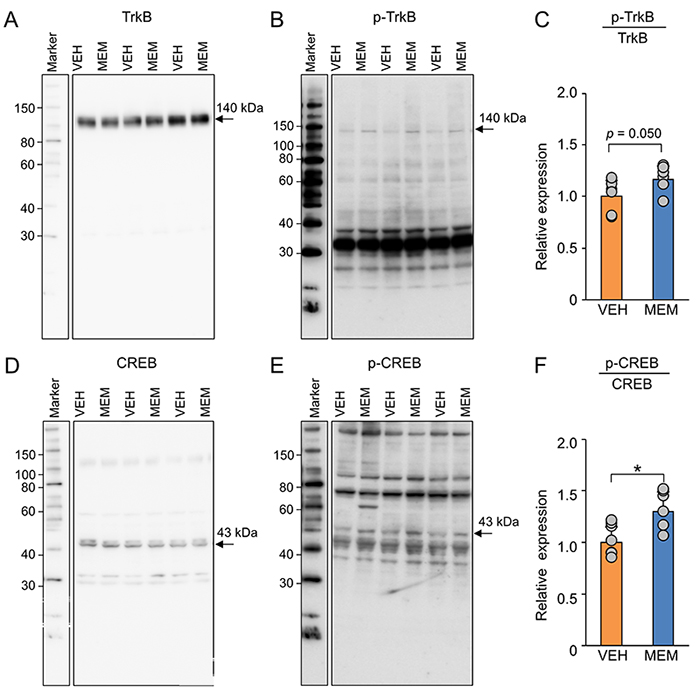
Various neurotrophic factors are involved in the regulation of adult hippocampal neurogenesis14. Neurotrophic factors have also been shown to interact with CSPG15. Based on these previous findings, we investigated the effects of MEM on brain-derived neurotrophic factor (BDNF)-related genes by RT‒qPCR. BDNF expression levels were increased by MEM treatment in both the dentate gyrus and CA1 region of MA mice (Figure 3A). In the dentate gyrus, MEM increased the expression levels of TrkB, CREB, NGF, and TrkA but not the expression levels of Akt, ERK, and CaMKII (Figure 3B-H). In addition, in the CA1 region, the expression levels of these molecules were not altered by MEM treatment.
The increase in the expression levels of BDNF-related genes in the hippocampus after MEM treatment led us to analyze the phosphorylation of BDNF downstream molecules by western blotting (Figure 4). The results showed that the ratio of phosphorylated TrkB and CREB was increased by MEM treatment in the dentate gyrus of MA mice (Figure 4). Together, these findings suggest that MEM treatment may activate BDNF signaling.
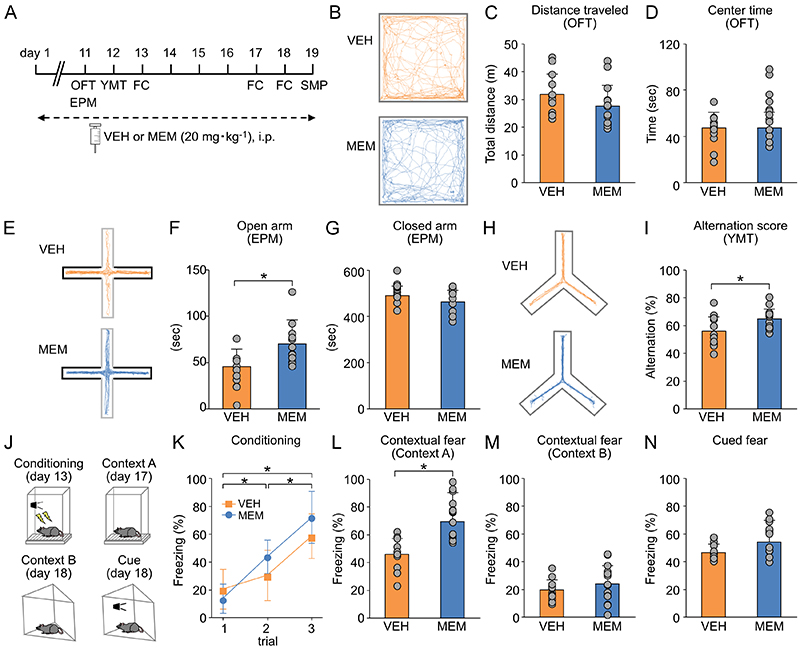
The effects of MEM on cognitive functions in MA mice were examined semi-comprehensively by a behavioral test battery (Figure 5A). MEM did not change the time spent in the center zone in the open field test (OFT) (Figure 5B-D) but prolonged the time spent in the open arm in the elevated plus-maze test (EPM) (Figure 5E-G) and improved the alternation rate in the Y-maze test (YMT) (Figure 5H, I). In the fear conditioning test, contextual memory, which is considered hippocampus, was improved by MEM treatment (Figure 5J-M). On the other hand, no change was observed for cue memory, which is considered amygdala-dependent (Figure 5N). These results suggest that MEM treatment may suppress anxiety-related behaviors and improve working memory and contextual memory in MA mice.
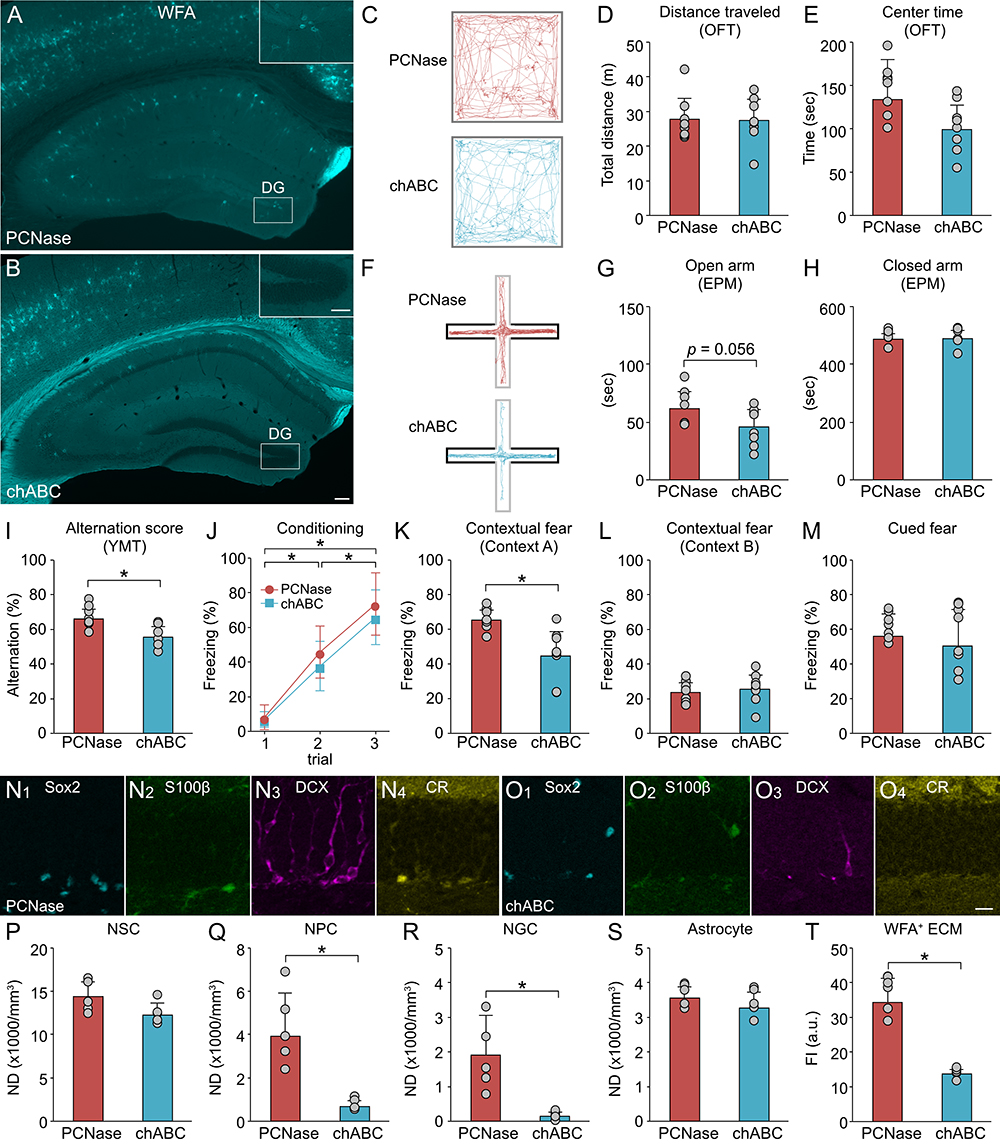
We conducted pharmacological studies to confirm whether the presence or absence of the ECM makes a difference in the action of MEM. In this experiment, chondroitinase ABC (chABC) was injected into the dentate gyrus of MEM-treated MA mice to degrade CSPG (Figure 6A, B). As a control, penicillinase (PCNase) was injected into the dentate gyrus of MEM-treated MA mice. There was no change in the time spent in the center zone in the OFT (Figure 6C-E). Meanwhile, CSPG degradation tended to shorten the time spent in the open arm in the EPM and reduce the alternation rate in the YMT (Figure 6I). In the fear conditioning test, hippocampal-dependent contextual memory was impaired by CSPG degradation in MEM-treated MA mice (Figure 6J-M). These findings suggest that the cognitive improvement by MEM is mediated by CSPG in the dentate gyrus of MA mice.
Using molecular markers, we investigated the effects of CSPG degradation on the neurogenesis in MEM-treated mice (Fig. 6N, O). CSPG degradation did not affect the NDs of NSCs and astrocytes but decreased those of NPCs and NGCs (Figure 6P-S). We confirmed that the staining intensity of WFA-labeled CSPG was decreased by chABC injection (Figure 6T). These results suggest that cognitive improvement by MEM in MA mice may be mediated by promotion of adult neurogenesis following increase in CSPG.
This study showed that cognitive improvement by MEM may be mediated by increased expression of CSPG in the dentate gyrus and the consequent promotion of adult neurogenesis in the hippocampus. In the future, we intend to identify the mechanisms that regulate the molecules involved in the biosynthesis and degradation of CSPG and to elucidate the underlying principles of cognitive improvement. We also expect that our efforts will lead to the development of innovative anti-dementia drugs targeting CSPG in the hippocampus.